LED Light Therapy
IntroductionThis article is about red and near-infrared light therapy for healing recent injuries and a few other possible things like fibromyalgia pain, dementia, retinal injuries, and wrinkles. This is closely related to (if not identical in results) to LLLT (low level laser therapy) but it usually uses LEDs instead of weak lasers. For example, you can shine a red laser pointer on a cut for a minute or two to see the pain go away. This article does not discuss SAD, psoriasis, acne, and jaundice which are helped by white or blue light.Inexpensive LED arrays: For the infrared healing wavelengths this article discusses, search for "security camera illuminator 850 nm" on Ebay, Amazon, and Aliexpress. WARNING: these are not medical devices. Buy these for medical purposes at your own risk. They do not have heat protection so using them on yourself may result in 2nd degree burns. With dark skin, the skin can get too hot in less than 2 minutes. For light skin, in 5 minutes or less. They almost always have a light sensor in the array that keeps it off if there is light in the room. It can be covered with black tape or disconnected by cutting one of its two connections to the circuit board. The ones that are "invisible" are 940 nm which is not the correct wavelength, only the ones with with a slight red glow are a healing wavelength (850 nm). These are stronger than most medical LEDs arrays. You may need to buy a 12 VDC power supply separately. For red search for "red led light traffic signal". There are blue LED flashlights for completely disinfecting cuts, scrapes, and acne in 5 to 10 minutes for faster healing and less pain. DO NOT LOOK AT BLUE LEDS! See also the halogen light section for very powerful $7 lights you may already have in your house that have all these wavelengths.  Red and near-infrared light are a "window" of wavelengths that are able to pass through tissue up to 1 inch deep (not 6 inches like some web sites claim). Red and near-infrared have beneficial effects on cells by "kick-starting" them into immediately creating more ATP (cellular energy) and increasing DNA and RNA activity. This effect has been carefully studied in many published reports since 1987. The positive effects occur only in injured cells. There is probably not much benefit to healthy cells. In the past, lasers were thought to be needed to provide the light, but it's been known since 1989 that LEDs are just as good. The ideal wavelengths are between 600 and and 900 nm, with the best results at specific ranges: 610-625, 660-690, 750-770, and 815-860 nm (see below). LED light arrays are a means to provide these wavelengths. Companies may claim lasers and pulse rates are important, but the only things are the wavelength and total amount of light energy applied. For example, 880 nm is a bad choice. Bright noon-time summer Sun only has half as much light energy as LED devices in the optimum wavelengths, but it covers the entire body (which is good for fibromyalgia). The advantages of LEDs over sunlight are: 1) LEDs can be applied at any time, 2) LEDs require only one hour instead of two hours for injuries beneath the skin, 3) LEDs don't cause sunburn. Halogen lights emit a spectrum of light that is very similar to sunlight (see this chart or this). Like bright sun, halogen provides an inexpensive source of "healing light energy" in the 600 to 900 nm wavelengths (see Halogen Lights section below), but the energy is not concentrated at the best wavelengths as in LEDs. My interest in light therapy increased a great deal when a halogen treatment returned my black-and-blue broken small toe to almost a normal color in 5 minutes. Pain level went from 8 to 2. I repeated the treatment 6 hours later when the toe turned blue and painful again and received the same benefits. Heat lamps have long been used to reduce pain. It was believed the heat provided a benefit, but now we know the near-infrared portion of the spectrum of heat lamps provides more benefit than the heat of the far-infrared. Conditions HelpedConditions that are probably helped
 How useful is light therapy? I view red and infrared light as equally beneficial regardless of the source (sun, halogen, LED, or laser) as long as the intensity and area of coverage are equal. I think 830 nm is best, but maybe 660 nm is better, and in either case, you can just make the less beneficial wavelength have a stronger light source. There is no theory that indicates the effects are different. They kick-start injured cells into making more ATP. I view red/near-infrared light therapy as beneficial overall as applying ice to injuries, keeping in mind that people greatly under-utilize ice. More important than ice and light for joint injuries is a lot of stretching, movement, and careful strengthening. I use light mostly immediately after an injury, right before applying ice for 5 minutes, and then repeat once every 6 hours for a day or two. Immediately after injuries, ice is more important. FDA allows advertising red and infrared for minor pains and mild arthritis. Red has been used to help halt dry macular degeneration which may have FDA approval. The following have FDA approval for specific devices (these are just those I know. I have not done a search): infrared 880 nm for diabetic peripheral neuropathy, 660 nm red for mouth ulcers in children on a type of chemo, "Titan" intense infrared device for wrinkles in a clinical setting, very intense (harmful) infrared devices for spots, and blue or blue/red for acne. There have been excellent results reported for tendonitis, shoulders, knees, small joints, and fibromyalgia. For most soft-tissue injuries beneath the skin, the pain goes from an 8 to a 2 (on a scale of 10) after an hour or two of treatment with good home-use LED devices. For exposed injuries like burns and retina injuries, only 1 to 10 minutes of LED light is used, depending on the device. Applying LED light for too long cancels the benefits, but the time of application is hard to determine. Too little light and there is little benefit, and too much light and there is no benefit. For injuries where the pain can be felt, I apply it only long enough to notice the maximum pain relief and no longer. The pain relief can be amazing in burns, cuts, and other wounds even if wound healing is not faster. The increase in the speed of healing can be directly measured in the injured retinas of rabbit. Stubbed toes can go from being purple-black to pink in one treatment. Serious injuries seem to benefit from 3 to 6 treatments/day (as the pain returns) instead of one treatment/day. Strangely, tendon and muscle soreness from working-out seems to not receive any pain relief, but chronic tendinitis seems to benefit greatly. From my experience in trying to help friends and family, it is beneficial only about 30% of the time in back pain. Companies have made various strange claims that I do not believe: yellow for wrinkles, green for cancer, and blue for wrinkles. Recent serious injuries benefit from several treatments per day. In hindsight, we can say "people have always known Sunlight is good for you". It seems intuitively clear to most people that Sunlight helps sick people and enables people to be more active. We know why from a chemical and biological viewpoint. Injured cells need the extra ATP to repair themselves. Healthy cells may generate extra ATP from the red and near infrared of sunlight to enable more activity in the daytime. If the ATP is not used (as occurs when resting in bright sunlight) it causes an increase in available glucose for which causes a slight "glucose high" that causes relaxation and sleepiness we all feel after 30 minutes in the sun. BTW, we know UV creates vitamin D that prevents colon, prostate, and breast cancer, greatly improves the immune system, bone strength, and reduces the incidence of osteoarthritis, having the potential to save 50,000 lives a year if people would get more Sun and wear less sunscreen. By comparison, skin cancer causes less than 10,000 deaths in the U.S. each year, only some of which are caused by too much Sun. The viens and arteries on the top side of the body are the only ones that can be seen after the light has passed through. It shows that blood blocks the light very effectively. This does NOT show the light can penetrate 1" inch through the hand. It shows how difficult it is for a device 10 times more powerful than the ones normally being sold can barely show up through the hand, in the dark, with a night vision camera. 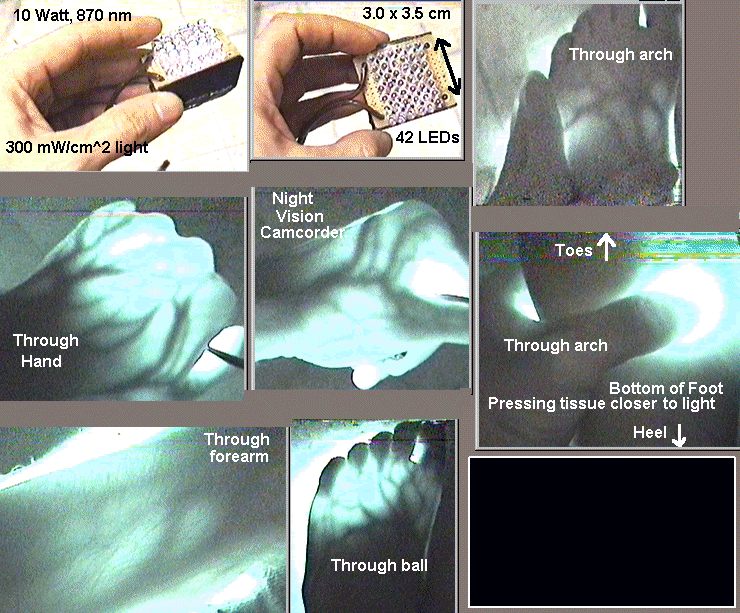 How does it work?The wavelengths from 600 to 900 nm pass through blood and water in tissue more easily than other wavelengths. About 35% of the energy in this range is absorbed by a specific "proton pump" (cytochrome c oxidase, CCO, "complex IV") in mitochondria. The light at 4 specific wavelengths "kick-start" the CCO pump into producing more cellular energy, ATP. The CCO pump is very similar in all animals because the key proteins were inherited from light-assisted bacteria that were part of the first mitochondria. The absorption spectrum of blood suddenly drops off to allow these wavelengths to pass through which indicates the evolution of hemoglobin may have been influenced by cells benefiting from these wavelengths. The immediate increase in respiration that Sun causes by this mechanism may help animals increase their physical and mental activity during the day, in addition to the heat provided by the Sun that promotes the release of oxygen (myoglobin also absorbs these wavelengths) and basic warmth. These wavelengths of the Sun can provide the optimum 4 J/cm^2 at a depth of 1 inch (using 1% transmission) after 1 to 3 hours of exposure in bright Sun, reaching all skin and a large percentage of muscle tissue in historically-thin humans with minimal clothing.Evolution Theory Support: There are 5 indications that the benefit of red and near-infrared light is not an accident, but a highly "intelligent" and natural result of evolution. The indications are: 1) The proton pump is the last in a series of 3 pumps which places it in possibly the best location to pull the food conversion process along by "pushing" the final electrons through the chain. This creates a chemical "pull" (which is a real electrostatic "pull") on the electrons further back in the chain, which can prevent reactive oxygen leakage if not too much light is applied. 2) The pump absorbs primarily the red and near-infrared light and the remaining sunlight wavelengths are blocked by water and blood. 3) The pump is the primary absorber of these wavelengths in the body, very roughly 35%. 4) Oxygenated hemoglobin has a very sharp decline in it's ability to absorb red and near-infrared which indicates hemoglobin evolved specifically to allow these wavelengths to pass through. The CCO pump has a longer evolutionary history than hemoglobin because it was inherited from bacteria that formed the symbiotic relationship in mitochondria. (I first wrote this here in 2006 to evolutionarily "explain" why blood is red). Decendents of these bacteria still exist as purple bacteria which are used in research on the CCO pump. 5) Night-time levels of melatonin, but not day-time levels, have been shown by Tiina Karu to completely inhibit the positive effects of infrared light. This indicates that melatonin and its wide swings over 24 hours could have evolved to not inhibit the benefits of sunlight. Although large animals may have only 10% of their cells effectively exposed to light going into their bodies, small animals that existed when blood first evolved had 100% of their cells exposed so it should have been strong evolutionary pressure to get blood "right" in terms of how it lets light through. In the "test tube", antioxidants negate the effects of the light, and the light negates the effects of oxidants. In some sense, this may be like the light is an antioxidant. Other reasoning indicates it helps by being a slight pro-oxidant in the same way exercise helps: the body's response makes it "stronger" in various ways. I focus on CCO, but a 2011 paper discusses the possible important role of other compounds (although I completely disagree with this paper's uncited wave-of-hand dismissal of combining wavelengths): Besides hemoglobin, the most common photoacceptors in the red to near-infrared range are also hemecontaining metalloproteins: myoglobin and cytochrome oxidase. Nevertheless, other molecules such as superoxide dismutase, cytochrome c, cytochrome b, nitric oxide synthase, catalase, guanylate cyclase, and the cryptochromes have also been shown to have photoacceptor capacities. Details on cytochrome c oxidase: As the CCO absorbs light, its two copper atoms are either oxidized or reduced to transport electrons that are required to help pump H+ to increase the gradient that allows for more ATP. This increases respiration ("food energy" is the input to the krebs cycle which outputs mainly NADH and FADH molecules that provide the energy for the electron transport chain, of which CCO is the last step). Calcium Ca2+, alkalinity (0.2 units) are increased which causes important secondary responses such as transcription factors that increase DNA and RNA activity. The creation of excess ATP (H+) may block the electron transport chain that may cause a leakage of electrons that cause toxic reactive oxygen species, but the body's response to milder oxidation from smaller doses is believed by many to be a primary benefit of the light. Daily moderate use of light therapy induces up-regulation of antioxidants like MnSOD to counteract the harmful oxidation of O2- in a manner much like moderate exercise. But, by using up all the electrons to pump H+ causes light therapy to increases NAD+ which is known to increase endurance as well as increase MnSOD. Over the short-term, heavy exercise depletes NAD+ but light exercise increases it (reference). Light therapy increases GSH (glutathione) which decreases H2O2 that is produced from the extra MnSOD that is converting O-2 to H2O2. Too much wide-spectrum infrared light, such as that received from 3 hours of bright sunlight, causes too much H2O2 which will increase MMP-1 (at least in the dermis of the skin) which some researchers think could cause photo-aging, but not cancer. BTW superoxide dismutase has an absorption spectrum in the 660 nm range. By creating an "electron drain" in complex IV (CCO), super oxide O2- may be decreased directly by an electrostatic pull on cytochrome c and thereby on complex III, preventing electron leakage that is believed to result in super oxide O2-. The chemical NO is prevented from halting CCO activity and this may explain the immediate pain relief. More electrons being transported to create ATP oxidizes ("alkalinizes") the entire mitochondria, increasing the ratios NAD+/NADH, NADP+/NADPH, GSH/GSSG and signaling important secondary effects such as transcription factors which signal more DNA and RNA. The idea that 600 to 900 nm wavelengths activate cytochrome c oxidase was first proposed 20 years ago by Tiina Karu in 1988. See her 2003 great summary for more about light therapy and CCO. cyt C transfers 1 electron (e-) at a time into the 1st copper atom (CuA) of CCO, which is then in the "reduced" state. That atom will oxidize (lose) that e- to the 1st iron atom (heme A). There are more atoms involved as the metal atoms are part of "complexes". There are probably different possible pathways for the electrons, so I speak roughly. heme then loses (oxidizes) the electron to the "bimetal core" of a copper and iron atom, CuB and heme A3. At least the heme A3 can hold 2 of the electrons while waiting for O2 and or CO(?) to get in place and for H+ to be transferred by simple thermal motion in the liquid environment (mostly water, which naturally has plenty of H+ and OH-, the log() ratio of which determines pH, roughly speaking). The H+'s are held inside the CCO until 4 are obtained and H2O is generated. So 4 e- are transferred into the CuA before a complete cycle is reached, but the initial transfer to and from the CuA of each e- is a cycle within the 4 e- cycle that is occurring at the bimetal core. 630 nm (apparently) brings the e-'s into CuA. 850 nm kicks them off CuA to the CuB-hemeA3 bimetal core. 760 nm seems to help bring them into CuB. 660 nm seems to kick them off CuB into the core reaction. CuB does more than simply receive and donate an e-, so 660 and 760 nm may do more than simply move an electron. 630 nm is the highest energy photon so 630 nm might be the most useful of all the wavelengths since it is the least likely to occur from a raw chemical energy viewpoint. It may also be the cheapest LED, so it is something I need to research. I see one paper saying 670 nm is more effective than 630 nm at low intensitis. It may not be an efficient LED or the transmission through skin may not be as good. Almost certainly blood evolved to allow it through. I believe all the energy levels of the electron transfers and other activity in CCO are on an energy level difference of only about 50 nm of photon energy, so the photons are absorbed and reflected from CuA and CuB atoms (more precisely, they "complexes") to a longer wavelength, which I hope to detect as an increase in the output of an 880 nm LED on the other side of my skin from application of 660 nm or 850 nm under different pulse conditions. I think most of the discussion on 660 nm and 850 nm is mostly from my writings on this subject. 630 nm, 670 nm, and 880 nm used to be the emphasis by companies making products like anodyne and warp10 (nothing wrong with warp10 except cost). I've been trying to get elixa customers to get them to change to 850 nm from 880 nm for years. My insistence over the last 9 years that there is no difference in the quality from the difference could also be wrong: perhaps the 760 nm or 660 nm are forcing a completion of the O2 to 2H2O reaction before all 4 e-'s have come into the bimetal core. 2013 update: since 660 nm act on the bimetal core and since NO competes with O2 at that location, 660 nm may release more NO than 850 nm.This could use up negatively-charge reactive oxygen species (like O2-)that are in the mitochondria allowing a less toxic a alkaline state. Similarly, 850 nm could do the same thing by preventing e- leakage out of the transport chain which causes ROS. So the comments I've seen that the benefit is from producing ROS is confusing because older papers and my reasoning say it should prevent it. However, if there is a build up of H+ when ATP is not being consumed by activity, then it could force more e-'s out of the transport chain that then cause ROS. So the combination of light therapy and exercise is a great idea, like our ancestors being out in the sun and working at the same time: not a coincidental situation, but evolutionarily instigated. So I am not sold on ROS theories. Certainly mild ROS from exercise helps. But I do not regard that as a reason to avoid light therapy and vitamin C before exercise. I think they should be used to help do a harder exercise until the right ROS is generated. Then do then again 2 hours later. Enough to stress the cells, but then give them the tools to repair themselves and repeat the next day, more frequently and/or harder than without the vitamin C and light. A guy called me once who was serious about using halogen lights and exercise at the same time. He wanted to build a treadmill with about 2,000 watts of halogen lights pointing at the runner. I encouraged him but emphasized he had to have water blocking and the people had to be naked on the muscle areas. I am building a swim-in-place indoor pool with a 3,000 Watt halogen flood light pointing into it. CCO absorbs energy from 600-900 nm (2.8 eV) photons and reflects them individually with a slightly longer wavelength (approx 50 nm longer), extracting about 0.1 eV of energy in assisting the 0.80 eV (not 0.43 eV) released from a molecule of ATP. If CCO in the body is able to absorb 5% of the 1E17 photons/cm^2 (30 mW/cm^2) in the 600-900 nm range from bright Sun over 0.5 m^2 of skin for 4 hours, then the body has gained 0.030*0.05*5000 cm^2 * 0.1/2.8 = 0.27 watts while using about 100 watts during those 4 hours (0.9 kcalories), making us 0.27% photosynthetic during those 4 hours. The light is directly photo-assisting in the creation of the ATP chemical energy. This does not include the calories absorbed from light that reduces the need for maintaining body temperature.  Below is a great summary from a professional 2010 source. What action does CCO modulate once it absorbs the energy from light? On the cellular level, LLLT may cause photodissociation of nitric oxide (NO) from CCO. In a stressed cell, NO produced by mitochondrial NO synthase displaces oxygen from CCO, which results in a downregulation of cellular respiration and a subsequent decrease in the production of energy-storing compounds, such as ATP. By dissociating NO from CCO, LLLT prevents the displacement of oxygen from CCO and thereby promotes unhindered cellular respiration [10] (see Figure 4). Increased CCO enzyme activity can be measured [11]; increased ATP production [12] and increased electron transport [13] also have been reported. The basic idea behind cellular respiration is that high-energy electrons are passed from electron carriers, such as NADH and FADH2, through a series of transmembrane complexes (including CCO) to the final electron acceptor. Increased cellular ATP produced by LLLT may contribute to the positive effects, both by raising cellular energy levels and by upregulating the cyclic AMP molecule (biochemically formed from ATP) that is involved in many signaling pathways. Oxygen acts as the final electron acceptor and is, in the process, converted to water. Part of the oxygen that is metabolized produces reactive oxygen species (ROS) as a natural byproduct. ROS (eg, superoxide and hydrogen peroxide) are chemically active molecules that play an important role in cell signaling, regulation of cell cycle progression, enzyme activation, and nucleic acid and protein synthesis [14]. Because LLLT promotes the metabolism of oxygen, it also acts to increase ROS production. In turn, ROS activates certain redox-sensitive transcription factors such as nuclear factor-êB [NF-êB] and activator protein 1, which leads to the upregulation of various stimulatory and protective genes. The ultimate effect of LLLT is likely to be produced by transcription factor activation, which modulates the host's downstream cellular and tissue responses (see Figure 5). Almost certainly, other mechanisms through which LLLT produces its effects are at play in addition to the one just described. For example, NO is a potent vasodilator via its effect on cyclic guanine monophosphate production. Cyclic guanine monophosphate is also involved in many other signaling pathways. LLLT may cause the photodissociation of NO from intracellular stores (ie, nitrosylated forms of both hemoglobin and myoglobin, in addition to CCO) [15]. LLLT promotes the synthesis of deoxyribonucleic acid (DNA) and RNA [16] and increases the production of proteins [17]. It also modulates enzymatic activity [18], affects intracellular and extracellular pH [17,18], and accelerates cell metabolism [18,19]. The expression of multiple genes related to cellular proliferation, migration, and the production of cytokines and growth factors also have been shown to be stimulated by lowlevel light [20]. LED Strength and DoseMost manufacturers and users of LED devices are underestimating how much time is needed to treat injuries beneath the skin. White skin blocks very roughly 90% to 95% of the incoming light, which most users are not taking into account. Most simple home devices need to be applied for an hour to treat injuries beneath the skin, but manufacturers and users very offten apply them for less than 15 minutes, so the technology works a lot better than most users realize. When injured cells are directly exposed to the light (such as in a test tube or the retina) esearch indicates 4 to 6 Joules of energy (J) applied to each 1 cm by 1 cm area (1 cm^2) once or twice per day is the best dosage. At 10 J/cm^2, all the benefits gained from the light can be negated, so not applying too much light is important. It's really difficult to know how much light gets through any particular person's skin to a particular injury. The best guide is to stop treatment when the pain has been reduced to as much as you expect. If the light is helping a particular injury, the pain will typically be reduced from an 8 to a 2. LED devices specifications should always include W/cm^2 so that the application time can be estimated. However, the LED arrays available are all about the same in terms of effectiveness. Paying more than $2 per standard LED is not needed because the LEDs are the most expensive part and cost only $0.15 each. Manufacturers are powering the LEDs the way they should so that all LED arrays using the standard 5 mm "bullet" type LEDs are the same, emitting 0.010 to 0.015 W (10 to 15 mW) per LED and usual spacing is 2 LEDs per cm^2 (20 to 30 mW/cm^2). A Joule (J) is a Watt (W) applied for 1 second. A watt is voltage times amps. So 6 J/cm^2 is the same as applying an LED device with a strength of 30 mW/cm^2 for 200 seconds (200 seconds x 0.03 W/cm^2 = 6 J/cm^2). The only benefit of stronger LED devices is a shorter treatment time. Ten to 20 times as much energy (Joules) is required to treat tissue that is beneath the skin, or 10 x 6 = 60 J/cm^2 because of the 90% to 95% light (or more) that is blocked by the skin and other tissue between the skin and the injury. For a 0.03 W/cm^2 LED device, 60/0.03 = 2000 seconds = 33 minutes at a minimum. This dosage can be applied twice a day and is not harmful to tissue. Dark skin may require three times more because it blocks very roughly three times as much light. Maybe 500 J/cm^2 is needed to reach injured cells 2.54 cm (1 inch) below the skin (4.6 hours at 30 mW/cm^2). For this reason, typical LED devices are probably not useful for injuries more than 1 inch deep, but to my surprise, they have been useful for knee and shoulder injuries (and are used by professional basketball teams). Higher doses could be dangerous, but I have successfully benefitted from 1,000 J/cm^2 with a halogen spot light supplying red to near infrared at 10,000 mW/cm^2 (100 seconds to get 1,000 J/cm^2).Research in animals has shown there is a limit to the intensity the cells need to receive, 4 mW/cm^2 in one and 15 mW/cm^2 in another. This means intensity at the skin surface for injuries beneath the skin might better if they are below 80 mW/cm^2 (assuming 5% penetration through skin: 4/0.05=80). Our ancestors have been exposed to 0.01 to 0.03 W/cm^2 of sunlight in the red to near-infrared range for up to 6 hours a day, giving an average daily dosage in the hundreds of J/cm^2 for very large areas of skin. People with fibromyalgia and arthritis will not a large reduction in pain when the painful areas are exposed to sunlight for a long time. This is strong support for the idea that 1,000 J/cm^2 is not unreasonable in a clinical setting for > 1 inch deep injuries that occur from trauma or surgery. I have found 100 J/cm^2 to reduce pains that are about 1/2 inch deep from an pain level of 8 to 2. I have not observed any harm from 30 minutes of 150 mW/cm^2 (270 J/cm^2) at 850 nm. LED strength in "mcd" is meaningless. The plastic bulb of LEDs can focus the light to a bright point that has a high mcd rating but as soon as it passes through the skin it's dispersed again as if it were never focused. The important rating is the power per square cm in units of mW/cm^2. A higher mW/cm^2 means less application time is needed. If the manufacturer did not oversize the power supply, the wattage of the power supply should be about 4 times more than the total light energy output of the LED array. The mW/cm^2 is the total light energy in mW divided by the length and width of the array in cm. Your cheek can barely feel the warmth after a few seconds of 30 mW/cm^2 in the 600 to 900 range and 150 mW/cm^2 can make dark skin too hot (> 105 F) after 1 or 2 minutes. Dark skin gets much warmer than light skin from LED devices because the melanin is blocking more of the light, so dark skin requires longer treament time. Optimum WavelengthsCertain wavelengths provide a better biological response. In short, CCO absorbs 4 peak areas of wavelengths (see figure below) in the 600-900 nm range that cover almost half of the 600-900 nm range. In an activated state, the CCO changes shape so that even more wavelengths are absorbed. It uses this energy to increase ATP and place the cell in an alkaline or oxidized state that results in many secondary benefits. From T. Karu, 1996 and 2005 This wide range of wavelengths is specific evidence for the general evolutionary argument that a wide range of wavelengths exactly like the Sun is the best possible exposure. However, there are three ways it might be possible to provide equal or greater benefit than the Sun for hypoxic or injured cells: 1) LEDs can provide injured cells with a larger amount of light in the beneficial range and at times when the sun is not available, 2) we can reduce the heat and thereby provide higher concentrations that reach deeper cells (the Sun is limited to about 1/2 to 1 inch of depth like most LED and laser units), 3) in the future an inexpensive device will be made that is specifically tuned to the CCO set of proteins, having a specific sequence of pulse times of specific wavelengths and pauses, forcing CCO through each step of its pumping action with minimal heat and maximum depth. A single wavelength may work as good as full spectrum by causing an electrostatic push or pull on neighboring electrons when moving only one electron (into or out of one of the two copper atoms in CCO). The electrostatic push and pull may cascade all the way through the electron transport chain. Complex II activity has been shown to increase even though it does not absorb these wavelengths. Many different wavelengths have been used, but very few studies have compared different wavelengths. The figure above indicates wavelengths 610-625, 660-690, 750-770, and 815-860 nm are the best wavelengths. Considerations other than how well they activate CCO are: 1) which wavelengths penetrate the best (see section on absorption), 2) which LEDs provide the strongest light output (keep in mind 850 nm has 30% more photons per watt than 630 nm), and 3) possibly 630 nm being usefully absorbed and reflected as (aka "converted to") an 825 nm photon to be used again. Inexpensive LEDs typically come in 630, 660, 850, and 880 nm with a hard-to-find (expensive) gap between 710 and 830 nm. (Often $1 for 830 nm and $0.05 for 850 nm). The peaks of the LEDs and optimum wavelengths are not exact, but spread out about +/- ~15 nm so there is an overlap of available LEDs and the biologically optimum wavelengths. The 630 nm LED can affect the 620nm peak in the chart, and 660 nm LED touches the 670 nm peak, and 850 nm is directly on one peak, but does not cover the nearby peak 820-830 nm as well.  Ability of Light to Penetrate TissueRed and near-infrared light penetrate tissue because they are not blocked by blood or water as much as other wavelengths. A doubling of the light intensity at any particular wavelength will double the amount of light energy that reaches a particular depth. Also, doubling the time of application will double the amount of light energy. So if you use a device that is half as strong, you simply have to apply it twice as long. Skin, fat, bone, and muscle all have different absorption and scattering coefficients that change depending on the wavelength which causes this to be a very complication subject, but the above two simplifying rules are good to keep in mind, as well as the following paragraph that goes into more detail. Knowing how much penetrates to the injury is very important because too little light has no effect, and double the "optimum" amount is suppoed to completely negates the benefits (possibly because of producting reactive oxygen). But it's impossible for anyone without the proper equipment and time to actually know how much light is penetrating for any particular injury within a factor of 2. So this section will go into detail, but it generally comes down to guidelines I've mentioned elsewhere in this document combined with stopping the light as soon as you see the pain reduction reach a maximum, which is the way I determine "what's best", keeping in mind pain relief may not be equal to optimum healing rate. But more or less, I believe maximum pain relief will be equal to optimum for healing. .630 nm and 660 nm red light penetrates arteries, viens, breast tissue, and pig lard a little bit better than 850 nm. But near infrared issupposed to be very roughly about twice as good as red at penetration of skin. My personal observations are that they are about the same. Melanin overrides all other differences between 660 nm and 850 nm: dark skin will get much better results and less heating of the skin from near-infrared than red. Through skull bone, 850 nm and 660 nm are about the same. Both penetrate water well enough that it is not a consideration. If a "FRACTION" amount of "INCOMING" light remains after a certain "DEPTH" of tissue, then you can determine the light intensity at a deeper or shallower depth of X*DEPTH (where X is a fraction if it is shallower, and larger than 1.0 if it is deeper than DEPTH). The amount of light at X*DEPTH is equal to INCOMING * [FRACTION]^X. For example, if after 1 cm of homogeneous tissue there is 10% of 830 nm light remaining, then at 2 cm there is only 1% light remaining. If a different wavelength, say 850 nm, has 15% light remaining at 1 cm, then at 2 cm it has 2.25% remaining. Notice that 850 was only 50% better at 1 cm, but more than twice as effective at 2 cm, so light penetration considerations are "exponentially important" for deep injuries. This is mathematically equivalent to the more complicated information below by the following math relation: 2.718^(-u*cm) = [2.718^(-u)]^cm = (fraction remaining at 1 cm)^cm where u="effective absorption coefficient" in cm^-1 units. My comments above do not assume the width of the array is larger than the depths under consideration, but the standard "absorption coefficients" discussed below do require this condition if they are to be used in the equations. If the source of light is a point source like a laser instead of an array, then multiply the "effective absorption coefficient" by 2 which means it will be much less effective at depth, eventhough laser may be much better at injuries immedaitely beneath the skin. This is because point sources decrease in strength by an r^2 factor as the strength is diluted as the "rays" from the source expand into an increasing surface area (m^2) of 3D space. Gravity and sound sources are other examples of this point-source geometrical effect. The next section goes into detail for a particular case of light penetration, the skull, and then it goes into more detail this section does not cover. Applying Light to BrainThe cortex is has many folds so that roughly only 1/4 of the gray matter (the neocortex) can be effectively exposed to light, 500 cm^2 out of 2000 cm^2. Where there are folds the light can reach more area than a straightforward spherical surface area calculation would indicate, but it can't effectively penetrate more than 5 mm of gray matter so that the deeper regions in the folds (where most of the area is located) are inaccessible. However, it is believed by some authors that by alleviating or assisting some of the "energy" demand (be it calories or some sort of increased computing efficiency) in the reachable areas allows more "energy" in other other areas. But certainly if a stroke area can be directly exposed to light, it should be.Light in these wavelengths penetrates skull bone a lot better than skin in most cases, and the scalp is some of the thinnest skin so the neuron cell bodies than are just under the skull bone can receive a substantial dose that increases ATP production that is beleived to be helpful in a lof of disorders if not improve thinking in general, as might be needed for summer days when humans were historically required to wake up, work, and think more than in the winter, at least for later humans with pale skin and male-pattern balding (vitamin D from UV needed more then the wavelength discussed here). LED light therapy for the brain is merely trying to replicate what was historically natural for humans during the summer. 850 nm penetrates and absorbs in the cortex neurons about twice as well as 660 nm and it penetrates to deeper neurons better, although a study on the retina showed 670 nm to work better than 830 nm. It is possible the quality of 660 nm (but not 630 nm) is different if not better than or synergistic with 850 nm, so the combination is not a bad idea. Here's an example of the math used in the tables below. Note: I use "cm^-1" in all my absorption discussion. Being careful not to use log() or 10^x when ln() and e^x is used for tissue penetration work, the equation for percent light transmitted. Also, it is always assumed the applied array is a few times wider then the depth you want to penetrate which is not the case for lasers. mW/cm^2 at depth = mW/cm^2 at surface * 2.718^( - effective attenuation coeff * depth) where effective attenuation coefficient = effective absorption coeff = ueff = aeff= sqrt(3*A*(A+RSC)) where A = ua = absorption coefficient and where RSC = u's eff= reduced or transport scattering coeff = (1-g)*SC where g is anistropy (average cosine of scattering angle) and usually g=0.9 I don't have SC (aka us) values, only RSC values which are 1533*nm^-0.65 for bone, which is about 18 from 800 to 1000 nm (although older papers range from 10 to 40 ). A gave 660 nm to be 27 instead of 22.5 that the 1533 equation implies. For SKULL BONE A = 0.11 1/cm for near-infrared 800 to 900 nm and red is probably only a little higher judging by the 2005 paper, and 0.27 by the 1994 thesis. The skull is 0.3 to 0.5 cm thick, giving 2.718^(-sqrt(3*0.11(0.11+19)*0.3 cm) = 46% for 0.3 cm to 28% for 0.5 cm of light makes it through the skull, out of the light that makes it through the hair and skin (so bald, light skin people will get a lot more than people with dark hair and skin skin). It appears somewhere between 12% and 30% of 660 nm makes it through the skull (not counting skin and hair blockage) and 20% to 34% at 850 nm, assuming 0.4 cm skull thickness, so there is not much difference. However, 850 nm makes it through skin better, especially dark skin. Treatment time may be able to be cut in half with 850 nm. 
Can helmet's cause Heat stress in the upper cortex?This will go into painful detail, so the rest of this paragraph is good for 99.9% of readers to skip. Looking at the table below, it appears reasonable that there will be many situations where 4% of the light energy will be blocked by the upper 1 mm of the cortex. Given that my device is capable of supplying 0.1 W/cm^2, this will be 0.004 W into a volume of cortex that is 0.1x1x1 cm^3 = 0.1 cm^3, or 0.04 W/cm^2. The cortex grey matter is about 2.6 mm thick and has a volume ot 470 cm^3 (Winkler 2010) and burns 95% of the 20 W the brain uses, which is 470/19= 0.04 W/cm^3, so my device has been heating the upper neurons of my cortex as much as my body normally heats the full cortex, more than doubling the heat in the upper 1 mm. Blood and cerebral flow and heat conduction to adjacent tissue probably reduce this heat a lot, but I wanted to show it's not obviously safe. We can withstand over 30 mW/cm^2 for 4 hours a day from the Sun, so 100 mW/cm^2 for 10 minutes must not be very dangerous, but anything more seems to have a potential for harm. Ultrasound which goes about 5 times deeper (5 cm) than light therapy has a safety limit that was said in one article to be 500 mW/cm^2 when applied to the brain another indication that 100 mW/cm^2 might be near a limit, but I do not know how well that ultrasound energy penetrates the skull compared to light energy. Strong ultrasound pulses less than 20 us might cause local heating so if I tried light pulses at 10 us on and 100 us off at 100 mW/cm^2 it might be much more dangerous. It is certainly unnatural. Ultrasound can be used as high as 1 W/cm^2 for the hip and I've very successfully used over 2 W/cm^2 light therapy with a 75 W halogen and water-filled snow globe for a broken pinkie toe. But these are not intensities I want to apply to my brain. The skin transmittances below assume baldness or gray hair and white skin
Let's say I want to improve the functioning of my mithochondria by applying light on a daily basis and taking CoQ10 and PQQ to hopefully increase the actual number of mitochondria by upregulating gene expression. I know for injuries the optimum dose for cells is 4 to 6 J/cm^2. A 2010 review paper on benefits for the brain says 1 J/cm^2 is what is fuond to be good for a mostly healthy cortex. I agree this sounds reasonable because the 4 to 6 J/cm^2 is for overtly-injured cells. But natural exposure to the Sun seems capable of providing a lot more. Maybe neurons can benefit from a full 4 J/cm^2 when spread out over a day, but be harmed if applied all at once with a 100 mW/cm^2 that covers 3 times the head surface area. I prefer to take 1,000 mg vitamin C after light therapy to the brain because no one can calculate the correct treatment time within a factor of 2 or maybe 4 which could cause excess oxidation and possibly not be healthy for neurons, even though the body's response to the excess oxidation might be how it provides more the benefit. Assuming a bald head and very white skin, I'll estmiate scalp penetration to be 5% for 660 nm and 10% for 850 nm and skull bone penetration times cortex absorbtion to be 20% for both. This gives 1% total penetration and cortex absorption for 660 nm and 2% for 850 nm. It's entirely possible actual penetration is 4 times more or 1/4 these numbers. To get 1 J/cm^2 at 2% penetration and cortex absorption, a typical 25 mW/cm^2 device that does not need a fan for overheating would require 2,000 seconds, a little over 30 minutes. This is also about the intensity of the Sun, but compared to 850 nm, the Sun's spectrum in the "healing range" is probably only 1/2 or 1/3 as effective in terms of penetration and activation of CCO and the Sun strikes about only 1/3 as much of the scalp as a full-head helmet. In other words 2000 x 2 x 3 = 3.4 hours in the Sun, which is less than a typical evolution type of working exposure in the summer growing season (at least if bald and white). So all this research, effort, and expense is just trying to replicate what is natural. See also the Engineering Design section for more details on creating a helmet for the brain. Pulsing is believed to improve results, but I believe this is only because the pulses are doing with less energy what longer treatment times could do with constant light. Most research still seems to be grossly under-applying light to humans in both soft-tissue injuries and the brain from a lack of understanding about the penetration issues. Studies on the human brain seem to be copying mouse, rat, and rabbit studies in the intensity, apparently completely oblivious to the fact that the animals' thinner skulls are allowing about 10 times more light through. It if this technology works for brain ailments, I am sorry to say patients will have to wait about 5 more years before the medical community catches up to what is written here. I myself have delayed getting on board for 5 years with this because I thought it was ridiculous to think the light could get through the skull. Articles concerning light penetration and the brain. Do not be misled by review papers by Rojas and Gonzalez-Lima. They list doses in various studies applied to the brain as "transcranial" when the most important ones they list were doses applied directly to the brain. Also they say (seemingly off-hand) that combined wavelengths cancel each other. It's not simply knocking CCO into or out of a simple redox state like the say. There are several redox locations that can be in states independent of the other locations. However, it might be that 830 nm and 630 nm at the same time or 760 and 660 at the same time could counteract each other because they operate on CuA in the first case, one for oxidation and the other for reduction, and CuB in the 2nd case. Excellent 2013 paper Be careful because they use logrithm base 10 instead of natural logrithm on p450 and got 2% light transmission for skull bone instead of the expected chapter 2 of a book 2010 paper 1999 paper 2005 paper (seems to have problem loading) 1994 thesis Wikipedia: Optical window in biological tissue Out of interest in this, I put together a helmet of LEDs as shown in the picture below to see if I could notice any effects. When using about 10 minutes in the morning at 75 mW/cm^2 output, it seems to have some energizing effect and maybe even helps me work better the rest of the day. It's 1,512 LEDs powered by 160 to 250 Watts of 660 nm and 850 nm. Light energy was about 40 Watts, 800 cm^2, 50 mW/cm^2. If 2% of the light made it through, that would have been 0.8 Watts entering the brain. For comparison, the brain uses about 20 watts, so it's a 4% addition which is probably safe. How short of treatment time is possible? I can turn the circuit up to 75 mW/cm^2 which about 100 mW/cm^2 reaching the scalp because my array has twice the area of my scalp and the LEDs are pointing inward so it is a kind of focusing. To get 1 J/cm^2 and assuming 1% effective light penetration, treatment time is 1/0.100/0.01 = 1000 seconds, about 17 minutes. Since it covers 3 times the area of the Sun and my chosen wavelengths are about twice as effective as the 25 mW/cm^2 "healing" wavelengths of the Sun and my unit is 4 times more powerful, this 17 minutes is like 17*3*2*4 = 408 minutes or 7 hours in the Sun. This is an argument to show this dosage might simulate what's natural and therefore safe and effective, although getting it all at once is certainly not natural.  This 1,512 LED unit of 660 nm and 850 nm was used by someone for a relative who has a case of Alzheimer's. I believe the setting was adjusted to about 150 W and I believe they both used it consistently for 15 minutes twice a day. The patient with "mild" Alzheimer's (according to son, 9/6/2013) has thin gray hair, so the light is able to penetrate well, with 2% penetration being assumed in the best studies. 150 W per 1500 LEDs is 100 mW/LED power supply, therefore about 30 mW light/LED at 1.65 LEd/cm^2 gives 50 mW/cm^2. Therefore each 15 minute treatment was supposedly a safe 0.9 J /cm^2 (less than the optimal 4 J/cm^2 for injured cells). This seems small, but I do not believe it was a weak dose for the cortex, especially if the cells are "nearly normal' and only partially compromised. They used a large fan to keep the exposed circuits and head relatively cool. This turned out to be too strong and there may have been a slight deterioration, at least in lifestyle. After they reduced the treatment time to 7 minutes twice a day Here are their comments: Received unit 10/15/2013. 10/18/2013: "We've both been using it twice per day at 15 minutes at the setting you dialed in. Morning and between 6-8 PM. It's hard to say at this point exactly what the effects are for [relative]. Most immediately it seems to brighten his mood, make him a bit more conversational. His sleep has been good. I quite like using it. I can't pinpoint exactly why.. but i do feel pleasant and calm for a couple hours afterwards....It can get depressing for all of us as he progresses further [downhill], but it's essential to try and stay positive." 11/6/2013: "It's been a few solid weeks of consistent usage now, and beyond some initial subjective effects, it does not seems like my father is benefiting. The only effect I observe and he feels is getting very tired/dozy afterwards. He also has begun to dread using it. We would like to return it if that is still satisfactory to you. It's too bad, as I quite like it, but i guess i could try building the simple IR array one in the future." 11/23/2013: "The 7 minutes twice a day has been better than 15 in terms of fatigue, but we still aren't seeing positive benefits enough to keep it. I'd like to send it back to you sometime next week..." 1/24/2014: "My folks were away for a few weeks traveling, mostly in Thailand and Myanmar. So I'm just getting him started again on the helmet. He's only doing 7 min once a day now. I think it's not quite enough. Maybe 10 min once a day? His mini mental state and other scores are holding." 4/7/2014: Notes from a phone conversation with son: After the initial month of using the helmet at 7 to 15 minutes twice a day as described above, he was more social and confident with words. Before using the helmet, his "mini-mental state" examinations had shown deterioration from 27 to 25 during about 4 tests over the course of a year (mild dementia), then improved to 28 (which is technically borderline normal) for 2 tests after the first month of using the helmet. His son's impression as of this date is that it has helped him from getting worse quicker, but nothing miraculous. However, only the first month was used at the strong dose, and he went on vacation and then into a methylene blue trial during which the helmet was not used. His hair got noticebly and definitely thicker and darker according to son, wife, and sister. His son estimates 15% to 20% thicker and 10% darker, and the sister noticed a difference in the hair before she knew about the helmet. We agreed on the phone that if the helmet had been used consistently for 6 months at the higher dose, then the results might have been very beneficial, if the mini-mental state exam results are "accurately indicative". The reason it was not consistently used at the longer treatment times is due to heat and comfort. So I recommended 3 treatments a day, maybe 7 minutes each, each treatment followed by 1 large vitamin C pill. Based on the theory of how it works, I would not be surprised if 10 minutes every two hours is an ideal treatment plan. I would guess maximum results (including improvement of the condition) to be seen in 2 or 3 months, and then holding steady after that. I am also using due to simply enjoying it, subjectively thinking it makes me more active or "awake". Making an LED helmetThis describes a different design that does not require soldering all the LEDs. It requires a familiarity with electricity and soldering. The LEDs are 850 nm infrared and show only a very faint red when turned on.KNOWN DANGERS: First of all, follow my writing at your and your loved one's own risk and sole liability. There may be eye damage from direct exposure to the LED arrays, and there is a real shock hazard to my methods (120 VAC and 48 VDC), and prolonged exposure to the head may harm the brain via excess oxidation and other unknown effects. Then there are unknown dangers and risks. 7 minutes 2 or 3 times a day is my best guess as the ideal for no hair and light skin. The time needed can vary a lot (10x or 20x) based on hair and skin darkness. By reading this paragraph and any other paragraph I write, you agree to assume complete and sole liability for what you learn here and for whatever mistakes I make in my explanation. If you can figure out how to structure a helmet with the circular arrays below, I want to see pictures of your finished product and I'll post the pictures to this page. The arrays have 48 LEDs per circular board which are 850 nm and it requires 12 VDC. These arrays are common, but use ones that have a smaller circular hole like the picture below. These "security camera illuminator 850 nm" arrays have a "CDS" light sensor to turn off during the day. You can cover the CDS sensor up to block light, or cut one of their leads to de-activate them. You'll need 28 of the $6.50 boards for 1300 LEDs for the whole brain area, so $200 is their cost. I wire them 4 in series so that a 48 VDC supply (3 or 4 amp from ebay or amazon, $34, see link below) can be used. 4 boards in "series wiring" makes a "set", then you would wire each "set" in parallel. It gets confusing and hard to follow the wiring, and you burn up 1 to 3 boards if you make a mistake. A 12 VDC supply (link below) does not have this problem and is therefore probably a better idea, but this requires a 15 amp 12 VDCpower supply requiring high gauge (14) wire from the power supply to boards and between boards which are all wired in parallel which is simpler "figuring" it and won't burn an array up. 15 amps at 12 VDC is only < 2 amps from the 120 VAC house circuit, so don't worry about the breakers. 36 VDC powering sets of 3 boards is also a possibility. Importantly, the power supply needs to have a voltage adjustment (a small knob on the circuit, exposed and hard to see, see image below) to maximimize light output at highest tolerable and safe heat. I use a box fan placed above my head and pointing down to cool my head. A box fan is needed or the power needs to be turned down and treatment time doubled to get same light dosage. This adjustable type of power supply will have exposed 120 VAC connections that can be covered with something like hot glue, but still a serious shock hazard to dementia and Alzheimer's patients. You'll have to use something like a cut old lamp cord to power it, ideally with a switch in the cord as the power supply does not include an off switch. 48 LED circuit 850 nm $6.50 each This is also on ebay since this link will probably go bad. Do a search for "security camera illuminator 850 nm 48 LED 5 mm". Choose board with smallest hole in center. 48 VDC 10% adjustable VDC, 7 amp power supply $34: Also on ebay. 12 VDC 10% adjustable VDC, 20 amp $26 Also on ebay.  Halogen light bulbs for the brain if you place a gallon zip-lock bag of about 1 quart water on your head then place a $10 500 W halogen flood light from walmart within 1 to 3 inches, then you should get a lot of light energy to your brain. When compared in terms of the energy from LEDs and laser, this should provide about 20 watts in the "mitochondria-active" range (1/3 of the 500 W comes out as light like the sun, and about 1/4 of that light is in the healthy range of 4 wavelengths, and about 1/2 of that is wasted in not being on a specific wavelength like the LEDs). The water is to absorb heat. This assumes the head is bald or shaved. It covers about 200 cm^2 so the intensity should be 20/200 = 100 mW/cm^2 which is twice as intense as my helmet but with maybe 4 times less coverage. Skin AbsorbanceThe question of what percentage of light is allowed through a particular tissue at a particular wavelength is very important, highly varied, and very complex. For example, there are 5 layers in the epidermis and dermis that have distinctly different absorption and scattering properties. All those variables change again based on the wavelength. An even bigger problem is that the research has such a large range in determining the absorbances, that the difference is a factor of 10. Additionally skin is 1 to 15 mm thick, depending on location, which results in another factor of 10.But the table below is the best anyone can do to simply the situation without leaving anything out, specifically for 660 nm and 850. Generally speakinb, 20% transmission is the most 660 nm can do through the thinner type of skin like the scalp of a skinny white person. 850 nm can get through twice as good. It seems most of a skinny white pale person can get 5% transmission plus or minus a factor of 10 depending on skin location. Perhaps 1/4 as much for dark skin and 10 or 100 times less than that if there is a lot of fat. But I don't trust the absorbance numbers for fat beneath the skin. My tests indicate half as much 850 nm light when going from about 0.1 cm fat to 0.3 cm fat rather than a 20x decrease as indicated below.  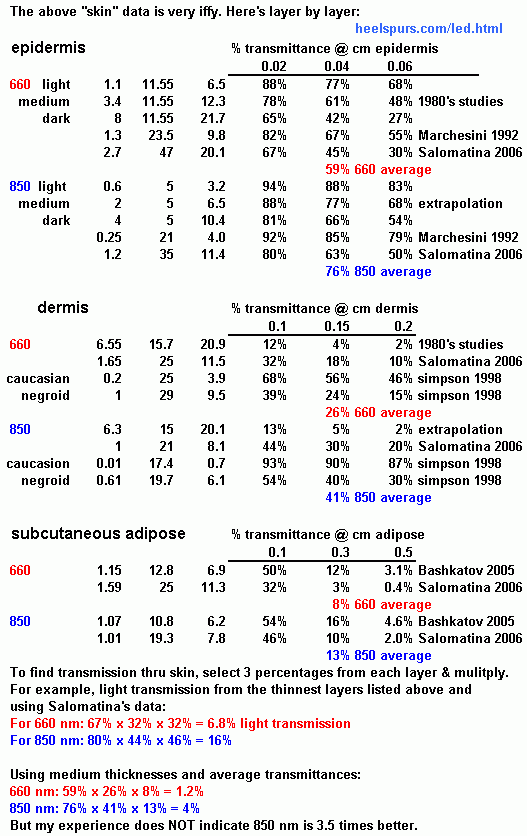
Great papers on skin absorbance: Bashkatov 2005 Bashkatov 2011 Salomatina 2006 Here's more data for skin at other wavelengths: caucasion epidermis and dermis epidermis and dermis 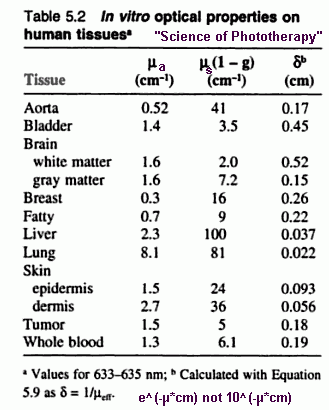 The graph below shows that wavelengths over 900 nm start to get blocked more and more by water.  The graph below shows not much light is able to pass through oxygenated blood (HbO2) when the wavelength is less than 600 nm.  Below is another interesting graph that shows that each mm of melanin in skin is very effective at blocking light, but that layer is very thin (less than 0.005 cm) compared to the small fiber collagen and hemoglobin in the dermis layer (0.1 cm).  For the best info on HbO and Hb in blood see this. and also S Wray. Water Absorption Factors, 200 nm to 990 nm Water Absorption Factor Chart Advice for Common InjuriesOf course, follow my advice or information at your own risk. I am formally educated in electrical engineering, not medicine.Physical therapy for most injuries is basically 1) maximum exercise without re-injury, 2) stretching, then 3) applying ice. Before the ice, electrical stimulation, ultrasound, and light therapy are the most common things that seem to be used. Vibration, massage, and stretching can be applied several times a day, especially for difficult-to-cure tendonitiis. I believe light therapy to be better than electrical stimulation and ultrasound, on par with ice. I believe light therapy can be used several times a day with recent serious injuries. I have not found it useful for skin injuries other than relieving pain, but I would certainly like to see it used in a burn clinic at low treatment times (3 minutes at 30 mW/cm^2 to get nearly 6 J/cm^2) since the cells are directly exposed to the light. I've read that light therapy research and acceptance by researchers was held back years because skin results were unimpressive and the skin is an obvious and easy thing to test against controls. How do you take a pictures of an internal injuries getting better? The author stated the lack of improvement over controls in skin injuries appears to be because the skin already heals as fast as possible, being the marvelous organ that it is. So the rabbit retina (back of eye) has been tested: they can take pictures of the impressive results compared to controls, and the retina is not skin. I do not know about increasing healing rate, but I know from experience really strong magnets reduce pain, especially for serious PMS cramps when using large rare Earth element magnets. Relief lasts 1 to 2 hours. I do not know how strong magnets "do their magic", but it's not a placebo effect. Search ebay for "security camera illuminator" 850 nm for effective "light therapy" devices. My halogen light section shows how to use common lights to work just as good or better than LED devices. They can be a lot stronger which means shorter treatment time and they are more "natural" like Sunlight with all 4 wavelengths instead of just 1 or 2. For the shoulder and knee, 2 light therapy devices can be used at the same time to opposing sides to cover a larger area to finish treatment time in 10 minutes rather than 20 minutes. But 1 device has been sufficient for me for relatively minor shoulder and knee injuries, in order to prevent them from becoming serious. Here's my advice for rotator cuff shoulder injuries, but you can adapt the following to any other joint injury:
Comparing LED WavelengthsLEDs typically some in certain wavelengths because the material science has discovered specific combinations of elements that results in the best efficiencies. So even if you tried to find LEDs that exactly match what research says are the best wavelengths for tissue response, you may suffer such a large loss in efficiency of the LED to emit light that it does nto make a difference (circuits can get only so hot, given the presence or absence of fans and heat sinks).660 nm verses 850 nm (update: 660 nm and 760 nm strike a different part of CCO that MIGHT cause it to pump more H+ with fewer electrons in the transport chain and thereby produce a more alkaline mitochondria. 850 nm ad 630 nm are more likely to simply speed up movement of electrons through the chain. ) 2013 update: since 660 nm act on the bimetal core and since NO competes with O2 at that location, 660 nm may release more NO than 850 nm. Wavelengths greater than 800 nm penetrate tissue a little better than wavelengths shorter than 700 (see Light Penetration section). The effect is much larger in dark skin which will benefit more from 850 than from 660. The question is complicated by different wavelengths having stronger or different biological responses. I don't know if one is better than the other, but I currently have a preference for 850 nm over all others. I am in the process of testing other wavelengths to see if I can find anything better than my best 850 device (shown below). 660 nm has a much weaker observed response on CCO, but experiments indicate it works. If 850 nm is better, it might be simply because 850 nm has 23% more photons per mW/cm^2 and CCO is activated on a per-photon basis. (Longer wavelengths have less energy per photon, so equal energy from 850 means more photons). I have not confirmed it, but it appears 850 LEDs are more efficient at emitting light energy than 660 nm and 630 nm, so from a practical viewpoint, circuits of a given style (such as no fan) using 850 nm are simply ABLE to emit more light energy, and LED device businessmen who turn into LED circuit designers, and even professional circuit designers, will not be able to see that the 850 nm spec sheets are saying they can get more light energy out of their circuits than 660 nm designs. The red and infrared spec sheets usually use different ratings (mcd verses mW/cm^2), and it's hard enough adjusting for the "viewing angle" of the LED as it affects the mW/cm^2 and determining if they are talking about 50% level or peak level, not to mention difficulties in conveting with mcd.  When taking penetration and benefits into mind, I do not know if one is better than the other. There are various LEDs in the range of 630 and 660. When I talk about 630 nm, I have 610 to 635 nm in mind, and when I talk about 660 nm, I have 650 to 670 nm in mind. 670 nm is closer to the peak response, so it's probably best to get that one instead of the 660 nm that I've usually used and found. 630 nm red is slightly orange and 660 nm red is a "deeper" red. Since 660 nm is almost infrared, the human eye is not able to see it as well. 630 nm red is used in key rings, traffic lights, and car tail-lights because it's 6 times easier to see than 660 nm (see the photopic response factor - chart ). The eye doesn't suddenly stop sensing light at 700 nm, but it is a gradual decline in sensitivity. You can see the healing and pain relief effect of light therapy by applying a laser pointer or a key ring light to small cuts for 2 minutes or to an arthritic finger joint for 20 minutes.  There are some companies that claim 880 is "the best" frequency, but it appears 880 is absolutely not as good as 850 or 830 nm LEDs. 880 LEDs are putting out frequencies in the range of 870 to 890 and are getting blocked 25% more by water absorption than 850 and the biological response to 880 nm is much less than at 850 nm (see Optimum Wavelengths section).  Measured light output is about 140 mW/cm^2 830 nm verses 850 nm Judging from the biological response to different wavelengths, it would appear 830 nm is the best of all wavelengths. But 850 nm may reach deep tissue better, and has a few more photons per mW/cm^2. The 830 nm LED is harder to find, and I don't know if its efficiency is as good as the more common 850. In the end, there may not be any difference between the two. There is other test tube work that showed 850 nm worked better than 830 nm for inflammation markers and my own experience has not been able to tell a difference for ankle, shoulder, and back injuries. 830 nm might work better by something like 30% but it's exponentially decreasing in strength by something like 10% in the exponential. At some point, the exponential effect on the 10% becomes larger than the 30%. This means that for the skin or cuts less than 2 mm deep, you would need to apply the 850 nm light 13 minutes instead of 10 minutes for the 830 nm. You save only 3 minutes by using the 830 nm, if it works better. But for deep injuries more than 1/2 inch, where you might need to apply the 850 nm for an hour, you might need to apply the 830 nm for 2 hours ... a huge difference. A similar effect might exist for 660 nm verses 670 nm, but I do not know which one penetrates better due to the complex nature of blood absorption in this range. 670 nm verses 830 nm 670 nm and 830 nm are probably better than 660 nm and 850 nm, but maybe not by a lot. I've seen one article that says 670 nm worked better on the retina than 830 nm eventhough more photons were available from the 830 nm. Since 830 nm is a lower frequency it has more photons per mW/cm^2, which means they could have given too high a dose because the CCO response is on a per photon basis, but I believe that is unlikely as they also suspected in the paper. Another thing to consider is that the 670 nm is a higher energy which can have more of an effect than 830 nm if there is a threshold of energy needed, which is probably the case. Another thing is that their operation on the CCO is different: one oxidizes it and the other reduces it. I have notice a 630 nm LED seemed to work a lot better than 850 on pain from a cut, but the energy from the 630 LED was more concentrated. I have enjoyed my LED helmet that combined 660 nm and 850 nm a lot more than my 850-only helmet, but it is impossible to know if it was just from the nice tingling of the scalp the red causes or if it was something on the cortex. 930 nm and above It appears any wavelength longer than 930 nm will start to have too much of its energy blocked by the water in tissue. See the non-ablative part of the skin section for how 1000-1500 nm can be used to burn the color out of spot and have other beneficial effects. Blue, Yellow, and Green See the skin section for information about how blue can help acne (it's really violet, near UV-A) . Blue is about 430 to 485 nm. Green is 510 to 565 nm. Yellow is 570 to 590. None of these penetrate deeper than the skin. See the skin section for how blue can help. There are some companies that claim yellow helps remove wrinkles. I haven't found any research on yellow for the skin that's not conducted by the people who profit from it.  Laser Light verses LEDsThere has been a lot of interest and money in low level laser therapy (LLLT) for healing, but there is no reason to believe that the coherent light from a laser is any better than LEDs, sunlight, or halogen lights. ( Unfortunately, authors of the last 10 years or so have twisted the historical meaning of LLLT to mean "low level LIGHT therapy" since lasers are fading away in importance in this area. ) Laser light might be more efficient since it may get through the skin more easily, but it does not penetrate more deeply once the light is beneath the skin, and cells do not know the difference: all photons are the same and the benefits are based on the action of each individual photon, not on bulk properties such as all the photons having the same polarity or coherency. The word "laser" has a superior marketing appeal for companies because it sounds interesting and mysterious. It also costs a lot which means patients can't do it on their own. These are the reasons there has been much more research in LLLT for healing than LEDs and halogens: companies and researchers have expected more profit. Light therapy is ancient and took on various new forms in the 1900's before lasers were invented. At least since 1989 definitive statements were being made in journal articles that lasers are not needed. To quote the most recognized researcher in LLLT, Professor Tiina Karu: "An analysis of published clinical results from the point of view of various types of radiation sources does not lead to the conclusion that lasers have a higher therapeutic potential than LEDs. ...The coherent properties of light are not manifested when the beam interacts with a biotissue on the molecular level....The conclusion was that under physiological conditions the absorption of low-intensity light by biological systems is of purely noncoherent (i.e., photobiological) nature....specially designed experiments at the cellular level have provided evidence that coherent and noncoherent light with the same wavelength, intensity, and irradiation time provide the same biological effect. Successful use of LEDs in many areas of clinical practice also confirms this conclusion." (Biomedical Photonics Handbook, 2003). Thankfully, Dr. Karu is a Russian Professor so we can expect her research to be more honest and scientific compared to U.S. medical research based on corporate profit. From a journal article: "...according to all available data, does not depend on the coherence of radiation." Reference: "Photobiological Principles of Therapeutic Applications of Laser Radiation" published by Yu. A. Vladimirov, et al in Biochemistry (Moscow) Volume 69, Number 1 / January, 2004.SunshineBright sun at midday in the southern U.S. in summer has as much energy in the red and near-infrared (600-900 nm) as LED arrays (about 29 mW/cm^2 - see chart below which has an error in saying '39'). But only about 50% of the light in the 600-900 nm range from the Sun is at the best wavelengths, so it requires mirrors and sunscreen to increase the amount of Sun to equal the best LEDs devices which have all their energy concentrated at the best wavelengths. If mirrors are not used, treatment time is doubled or tripled to get the same benefit as a good LED device, but then getting hot becomes a problem if you don't have a fan and a mist of water to keep cool.

 Halogen LightsHalogen lights are the strongest and least expensive light therapy, having approximately 35% of their light in the 600 to 900 nm range, being the man-made light source that is closest to the Sun because the filament temperature can get closest to the Sun. "Halogen" refers the inert gas (like iodine or bromine) that surrounds a tungsten filament that keeps it from burning out at higher temperatures so it can have a whiter color. Tungsten filaments are also used in common incandescent lights and heat lamps at lower temperatures. Incandescent and heat lamps will work almost as well if water is used to block the heat. I often use a 75 W halogen spotlight size PAR30 ($7) or a 100 Watt halogen flood light size PAR38 ($7) from Lowes or Walmart and a zip-lock bag filled with water to block the far-infrared heat. Follow my suggestions at your own risk. A snow globe filled with water can act like a lens to focus it to make it more intense, but that is not needed with the spot light. These lights are the standard outdoor lights you use on the corner of houses, although some are incandescent instead of halogen. You lay the zip lock bag of water on the injured knee, shoulder, ankle, elbow, wrist, finger, etc. Then hold the light as close to the zip lock bag without touching it. Shine the light through the water at the injured joint or other tissue. For the knee, kind of to the side or bottom so it gets on the side of the knee cap instead of directly on it. Shine it for 10 minutes once a day for most injuries. Fingers and other injuries that are not too deep need only about 5 minutes if the light is so strong you need sunglass to look at the skin surface reflection. This is going to be better than $100 to $1000 worth of LED or laser light therapy. I thought I was the first to figure this out, but it seems a small group of German researchers have known about it at least since 1995 (Hydrosun). My 75 W light is powered by a lamp cord that has a switch in the base, but you can also use a small lamp with the shade removed. There are several dangers to this, so I do not recommend anyone try it without strong caution. For example, the snow globe can focus the light too strongly and quickly burn the skin. The super-bright white light from the reflection off the skin can damage the eyes, so I have to wear sun glasses. The base gets hot, and setting the light down can burn tables and carpet. Dark skin burns very easily with this because it blocks all the visible light energy in the surface of the skin, concentrating the energy in a small volume of tissue. But here's what I use. Pretty much the same thing for $10 instead of $1000's(?). Add red food coloring to the water if you want to keep the spectrum better restricted to 600 to 900 nm. The most common red food coloring dye has a perfect absorption spectrum for this.   It's difficult doing pubmed searches to find others using halogen lights for healing, but you can find some articles by searching for Super Lizer or SuperLizer, Bioptron, and HydroSun. Light Therapy Bed After reading my comments about using halogen lights as a "healing" bed and for the winter blues, someone in Canada in winter 2007-2008 did a great job of implementing the ideas. I had a picture here of my "light bed" and he copied it, doing a better job (see picture below). It uses $5 full-length mirrors, $5 halogen 500 W flood lights, pyrex glass baking pans, and food coloring. The water in the pyrex pans is to block the far-infrared heat. The water can get nearly boiling hot. Use pyrex, and even with pyrex, do not blame me if boiling water spills all over your body. Use sunglasses because the blue of strong halogen lights at close range damages eyes. You do not have to use water, but you'll have to get further from the light because of the heat and take your sun bath a longer time.A lot of light energy comes through which warms you up. The food coloring in the water in the pyrex pans blocks visible light energy but still lets the red and infrared through. He actually found the spectrum from the manufacturer of the standard red food coloring which showed the proper wavelengths are allowed through. The food coloring is not needed (I wasn't able to tell much difference in the heat, and the Blue/green/yellow wavelengths are very good for the skin). The six 500 W flood lights use a total of 3,000 Watts and would need to be on two different electrical circuits in most U.S. houses without tripping a breaker. It's very calming and can put you to sleep quickly as if you're on the beach....it definitely feels as if you're on the beach. The only bad thing is that there is no U.V. for generating vitamin D which could prevent 50% of breast, colon, and prostate cancers in the U.S. - skin cancer is unimportant by comparison. 2010 update: He emailed: "About 20 minutes on my back then 20 face down.... Kind of makes me really relaxed and sleepy too . . . similar to eating turkey." 2011 update: He reports: "It heals scars and keloids - not immediately, but gradually. Also moles disappear over time, even ones I've had from birth." Email pictures to me at scott@heelspurs.com if you build a similar set up, and email me after a month and once a year to let me know how it's going so I can post results here.  Here's a simpler set up that works great in the winter. 
What would it take to do this with LEDs? To cover from the knees to the face, top and sides, about 10,000 LEDs running at 50 mW each with normal spacing of 1.65 LEDs/cm^2 (10.6 LEDs/in^2). Power supply would therefore be 500 W. With fans, you can run the LEDs at almost 100 mW each and use half as many (spaced further apart). The 5,000 LED array shown above (with my black New Balance shoe) cost $750 for the LEDs. Someone on alibaba.com is probably already selling this type of healing bed. They should target the 15,000 tanning salons and 50,000 chiropractors in the country. Email me if you find a good one. Halogen lamps will produce light like the Sun and it can provide more light energy in the healing (tissue penetration) range of wavelengths than regular incandescent and heat lamps. This will be much more energy than LEDs can provide and the energy will be spread out over a larger range of wavelengths (see chart above comparing LEDs and Sun). The halogen is closer to the Sun's natural spectrum. Halogen lamps usually have glass covers that block UV light so that desk lamps do not cause sunburn to hands. The strong blue wavelengths of halogens can be very harmful to the eyes. As with typical LEDs that have about 20% efficiency in converting power input to light output, and as with the wide-spectrum of the sun, halogen lamps also put out about 28% of the energy they use as light energy in the tissue penetration range. So a 75 W Halogen spot-light that concentrates 80% of its light in a 10x10 cm area will produce 75*0.80*0.28/10^2= 0.168 W/cm^2 = 168 mW/cm^2 of light intensity in the tissue penetration range, but the heat from the far-infrared in the skin will be too powerful to keep it there for more than a few seconds. This is about 3 times the best LED array and 5 times the healing range of sunlight. About half of this, 84 mW/cm^2, is near the four specific beneficial wavelengths. To get as much light from a halogen as from the sun, you can compare the heat you feel from a halogen to the heat you would feel from the sun and the healing dosage should be almost as much. Plexiglass can block some of the far-infrared that heats the tissue. Well-designed LEDs will not have the heat problem at all and are not supposed to be harmful to the eyes (I'm still researching it) which are two important reasons they are being used. LEDs are more powerful over a short range of wavelengths which appears to be just as beneficial as having the wattage spread over a wider range of wavelengths as occurs with halogens and the Sun. Halogen lights contain a lot of blue light and are very dangerous to the eyes. Comparing halogen, incadescent, heat lamps, and the sun:The Sun, Halogen lamps, incandescent lamps, and infrared heat lamps all emit light based on the black body radiation principle (see this excel spreadsheet if you want to calculate energy in a specified range of a black body spectrum). Halogen lamps have a curve half way between the ones shown for incandescent and the Sun (see this chart). The Sun and halogen lights have about 28% of their energy in the 600 to 900 nm range. Incandescents have 15% to 21% and heat lamps have about 10%. To produce light, halogen, incandescent, and infrared heat lamps heat up a spiral filament of tungsten metal. The filament "incandesces" which means it produces light by black body radiation. A halogen gas can allow the filament to get hotter than regular incandescent bulbs. Heat lamps are the same as incadescent lamps but their long filament is operating at a cooler temperature so that it produces more far-infrared. They operate at approximately the following temperatures: Sun - 5780 K, halogen - 4100 K, incandescent - 2800 to 3200 K, heat lamp - 2400 K. Energy in the far-infrared is easily absorbed by water in the skin, concentrating the light energy in the skin that causes pain from heat sensors.   Light Therapy and CancerI can think of four possibilities in regards to cancer and the use of red and near-infrared light:
Wikipedia has an excellent introduction to fermentation that is directly related to my discussion here: Fermentation takes place in the absence of oxygen, when the electron transport chain is unusable. It is used by the cell not to generate energy directly, but to recycle NADH into NAD+ so that glycolysis can continue, as long as glucose is present. The [ATP] energy generated by the glycolysis-fermentation pathway, a form of substrate-level phosphorylation, is small compared with that of oxidative phosphorylation. Fermentation consumes NADH, which in aerobic conditions might have been used to generate [ATP] energy in the electron transport chain. For that reason, cells generally benefit from avoiding fermentation when oxygen is available.I've also written an article on nutritional supplments and cancer. Pulsing LEDs for Great Improvement?On October 5, 2013, I published that day's version of this section to intructables.com as a creative commons idea so that no one can use these ideas for commercial purposes without my permission, but anyone is free to use and modify these ideas if they share. This is overly long and wordy like the rest of this article, but I am just writing to get my thoughts down for others to consider rather than for full or clear understanding by the majority or accuracy or all.Side note: When I mention 850 I am also thinking about 830 nm which is supposed to be better, and when I mention 660 nm, I am also considering 670 nm which might be better. It might be good to combine (in the same pulse time) 850 nm or 630 with 660 nm or 760, but not 850 with 630 or 660 with 760. This is because in the last two cases they might counteract each other. This is because 850 and 630 operate in an "opposite" manner on CuA , one for oxidation and the other for reduction, and 660 and 760 operate similarly (i.e. oppositely to each other) on CuB. I have historically been against pulsing LED light, but after thinking about it some more, and realizing the main problem with LED therapy (takes too long for weak devices that have an easy FDA approval process), I am open to the idea of pulsing...if it is done right. I'll first repeat my previous skeptical comments in the paragraph below, and then go into the details about the possiblity that good pulsing offers. Some companies claim pulsing the light is important, but it's a complex subject. Strong pulsing reaches deeper tissue, but it is less efficient at reaching depths in terms of a "total light energy applied" basis. Strong pulsing is necessary because if the "off-time" of the pulse is 50% and the "on time" is 50%, then the pulses will have to be twice as strong to deliver the same amount of energy in the same amount of time as a constantly-on device. This is a complex issue that would require a lot of research. I would not trust any company to know what the ideal pulses are. The different wavelengths for "healing" are all using the same principle: simply increasing ATP. So there is no theory to support a difference in the quality of the result based on wavelength, only the quantity (830 or 850 nm might be best). However, there was a test tube study that showed 850 nm did better than 830 (despite the graph way above) and that 660 and 620 nm did not reduce inflammation markers as well as the 830 and 850 nm. There may be many other articles out there that claim there is a difference, but I am skeptical. (update: 660 nm and 760 nm strike a different part of CCO that could cause it to pump more H+ with fewer electrons in the transport chain and thereby produce a more alkaline mitochondria. 850 nm ad 630 nm are more likely to speed up movement of electrons through the chain. ) I have not edited the rest of this section to be brief, and I want to show my reasoning concerning a complex subject where my knowledge is changing, so bear with me. Research in animals has shown there is a limit to the intensity the cells need to receive, 4 mW/cm^2 in one and 15 mW/cm^2 in another. This means intensity at the skin surface for injuries beneath the skin might better if they are below 80 mW/cm^2 (assuming 5% penetration through skin: 4/0.05=80). If this effect is NOT due to heating, then really strong pulses may not be ideal. For example, my 50 microsecond on times and 250 microsecond off times with an average intensity of 30 mW/cm^2 are 180 mW/cm^2 during the 50 microseconds. On the other hand, there may be a threshold effect for the intensity such that you need at least 4 mW/cm^2. In this case the pulses would reach deeper in the sense that even doubling the time of application for a device half as strong would not show benefit as it would normally do. I have not found any patents or research papers that have directly proposed or tested these ideas. Specifically, there are a lot of tests with longer pulse periods or longer duty cycles, but according to my thinking on the subject, those should not have seen the big improvement I'm going to describe, and they usually don't. The patentable idea is this: 600 nm to 900 nm LED and LLLT wavelengths can be used to increase beneficial healthful effects with less heating in the skin and circuitry by having pulse-on widths less than 250 microseconds with duty cycles of less than 50%. Ideal pulses might be as low as 5 microseconds "on" to 50 microseconds "off". For even greater effects, different wavelengths can be used together on in a sequence, but still have a primary characteristic of total light on being less than 50% duty cycle. I can easily see that sequential pulses of 670 nm, 620 nm, 760 nm, and 830 nm may more efficiently activate CCO than simply constant-on light that I normally promote. Each of the 4 wavelengths correspond to when one of the 2 copper atoms is either oxidized or reduced. When a particular CCO complex is pulsed into action with light, then according to the way CCO works as a pump, it will be a while before it is ready for another pulse and therefore more light would be wasted if it is kept on through the whole CCO cycle. Strong light pulses should force many if not most of the CCO's to be in a kind of sync. The duration of the pulses to be most efficient should be on the order of 1 to 100 of microseconds and the best delays between pulses will be between 20 and 1,000 microseconds. The difficulty is knowing these on and off times, and the sequence of the different wavelengths. Pulses are not an improvement on the way constant-on devices work, except to allow a shorter and deeper treatment with less heat in the skin and electronics. The main problem with LED therapy is that if you apply 180 mW/cm^2 of red and/or near-infrared light, people with white skin get too hot (>105 F, FDA rules) in about 3 minutes which is before the optimal treatment time for beneath-skin injuries (up to 10 minutes). Dark skin will get too hot in 1 minute at 180 mW/cm^2 (this is more of a problem with red than with infrared because melanin absorbs red more). My personal devices are 180 mW/cm^2 and I move them around or pause a few seconds to prevent too much heat in the skin after 5 minutes. It is a directly proportional correlation between reduced treatment time and the strength of the device. A device with 300 mW/cm^2 applied for 5 minutes appears to have a benefit that is indistinguishable from 30 mW/cm^2 applied for 50 minutes, but heat is a problem if someone where to try to sell such a device, and there is no easy FDA approval process. CCO is a ~10 stage pump with time periods between each stage. It's key to all animal life, but they've hard a hard time over the decades in trying to know the shape changes it goes through and the timing. If someone can list the stages with the time periods between each shape change, then I could build an LED array with the 4 wavelengths to kick the pump at the exact moment when needed, and thereby reduce the amount of time and energy needed to supply a treatment. With pulses like this, more of the light is getting blocked at any depth, but at the same time it is flooding the upper layers so that after saturation the pulsing actually enables the excess light to go much deeper because the pump seems to be the cause for a lot of light not going deeper. This may be way pulsing helps. However, in my own tests on the amount of light going through the skin between my thumb and index finger, I could not detect any increase or decrease in light penetration based on pulsing at various rates verses constant, and therefore I can't claim that neither synchronization or flooding occurs. But my test was far from being careful enough to be conclusive. I used 850 nm 660 nm with a detector and an oscilliscope. Typical LED's that do not need a fan for cooling (and can easily get FDA approval with a letter) emit about 30 mW/cm^2 and seem to have few heat problems even for dark skin (a timer for turning off is needed). So the idea is that since it takes CCO about 10,000 microseconds to complete one pump cycle (averaging 2,500 microseconds per electron, see below), and since it only takes on the order of 10's of microseconds to transfer electrons between the 4 metal atoms, and since it takes only 1 photon per metal atom for activation, then it should be possible to sequence strong pulses and have a long "off" time between pulses, so that the LED circuit and skin do not experience heat, and yet the tissue will get a treatment as if the device were 10 times stronger (assuming a CCO cycle can be sped up from about 10 ms to 1 ms, as my very limited knowledge suugests). In other words, the light pulses can "resonate" with the CCO cycles, speeding them up with less heat. It appears from the CCO pump cycle that the off time(s) can be at least 10 times longer than the "on" times with no decrease in effect from constant-on, so it should be possible to get a 300 mW/cm^2 benefit from a 30 mW/cm^2 device, which means 5 minute treatments for the shoulder and knee compared to 1 hour with a constant-on device that will not reach as deep. I mention a sequence of different wavelengths, but even 1 wavelength of 620, 670, or 830 nm may be enough (although not ideal), since I know the wavelengths by themselves work, and the CCO complex can't (for the most part) use more than 1 photon of a particular wavelength at a time, and that it is very roughly 50 to 1,000 microseconds before it needs another photon. My method is to first find out how long human CCO typically takes to complete a cycle. The only number I have is 10,000 microseconds for bovine heart CCO to convert one molecule of O2 (another paper agrees). I begin by dividing by 4 for each of the 4 electrons that need to be transferred from cytochrome c during a cycle as an estimate for how long it is before the next electron is needed. This is really too long because it appears 2 of the electrons are transferred very close together while the rate-limiting step(a) is (are) between the other 2 electrons, but I'll shorten the timing in a later paragraph. I want to give a strong pulse of all three wavelengths so that any spare electrons on each of the 3 metal atoms can be "kicked" to the next step. So the pulses should be about 10,000 / 4 = 2,500 microseconds apart. But I want make the ATP production cycle go faster in the injured cells than what normally occurs in resting healthy cells. I think injured cells and "exercising" cells need to and can produce a lot faster if the nutrients are present and needed, so I'll wildly guess they can efficiently run 10 times faster than normal cells. In other words, instead of 2,500 microseconds between electrons and the needed light pulses, I'm shooting for 250 microseconds. If this is too fast, then some light energy might be wasted, but not nearly as much as constant-on. Note on the rate-limiting steps: the transfer of an electron from cyt C all the way to the bimetal core can be less than 100 microseconds and yet it takes a 4-electron CCO cycle roughly 10,000 microseconds instead of 400 microseconds. The rate-limiting step(s) could be connected to each other and occuring during the electron transfer between hemeA to the hemeA3/CuB bimetal core, waiting on molecular structural changes in the bimetal core, or waiting on an electron to transfer from cyt C to CuA. See the images below. Side note: When I first get into sunlight, I can immediately see at least a 25% increase in my breathing rate, well before there is any extra warmth. The Sun is providing about 30 mW/cm^2 of the ATP-generating wavelengths, 95% of which is blocked. Maybe only 20% my cells are being exposed (~ 1 cm deep on 1/2 my skin surface). This implies 0.25*20/0.20 = 25 times increase in those sunlight-exposed respiring cells. This increase in breathing stops if I do not engage in exercise and the ATP builds up to block further H+ pumping. This reasoning indicates exposure to these wavelengths while exercising will help increase the ability to exercise. Running and lifting weights on the beach is an ideal way to do this, especially if sunscreen has helped prevent the skin from tanning which would block a lot of light. The heat itself should also help the conversions (which is increased by tans). As a 3rd example, I know 10 minutes of strong LED therapy decreases pain for at least 2 hours. Those injured had to get a LOT more ATP, not just a little, and someone did something useful with it. 120 minutes divided by 10 = 12 times more "ATP" (this is iffy because I do not think this much ATP can be stored.) end side note Getting back to the previous paragraph: I then divide my 250 microseconds per electron transfer goal by 10 as the total amount of time I want the light pulse to be on for each "accelerated electron cycle" (25 microsecond pulses, 250 microseconds apart). Short pulses that are only 1/10th as long as the off-stage can be 10 times more powerful in terms of light coming out without burning the LEDs up or heating the skin. The hope is that I can thereby force all the CCOs to be "in sync". I know 30 mW/cm^2 does not cause too much heat in skin or the circuitry, so I should make these pulses 300 mW/cm^2 which is only 30 mW/cm^2 averaged over the cycle. I know from experience 300 mW/cm^2 does not appear to be "wastefully intense", but it should be getting close, so I do not wish to try 20x stonger pulses (and LEDs can't handle it). 50 microsecond pulses half as strong still spaced 250 microseconds apart is also reasonable (shooting for 5x instead of 10x). LEDs are about 25% efficient, so I know my pluses will require 1,200 mW/cm^2 electrical energy. A 1.5 microsecond pulse every 150 microsecond might be plenty (as they tried in tomato plants at 668 nm for photosynthesis which uses similar biological structures. It seems what I am attempting was tried in this paper in tomatoes and they failed. 10 to 20 microsecond pulses every 100 microseconds may be best because 100 microseconds is about the speed at which 2 of the 4 electrons in a cycle can make it through (the other two are probably much slower, see below). "Kicking" the metal atoms with light creates an electrostatic *pull* on the cyt C, and a *push* on the "bimetal core" where the main enzymatic reaction with oxygen and water is occuring. (( Side Notes: Interestingly, in the plant article above it took 10 photons to convert 1 molecule of CO2 into O2. If every metal atom step of CCO doing the reverse process of converting O2 to CO2 required a photon (850 nm for CuA, 620 nm for heme A, and 660 nm for heme A3 to CuB) then it would take 12 photons (3 wavelengths times 4 electrons). In the plant, 2 millisecond pulses every 200 milliseconds (1,000 times slower) caused photosynthesis to be cut in half. )) Since 3 LED wavelengths are hard to crowd into 1 unit, I could try 830 nm and 660 nm (or 620 nm). I'll probably try 830 nm by itself since I have the most experience with it being "constant on" and I need to compare. 660 nm instead of 620 because eventhough it does not have as much absorption, it is uniquely active at the core during the slowest part of the cycle. I'll update this paragraph when I find out how well it works. It takes some time because I have to wait on several good injuries in the family to test it on (I'm writing this June 2012, so maybe I'll know by January 2013). There are numerous other studies that show a little or no benefit from pulsing, but only a few with < 500 microseconds as required by theory, and none had detailed enough abstracts to see if their duty cycle was correct as predicted by CCO theory. The pulsing I'm describing would hopefully show 5 to 10 times benefit (more cell attachment in test tube) from equal light energy. Ideally, it might be better to do a *sequence* of < 10 microsecond light pulses of different wavelengths instead of all wavelengths at once. For example, a 660 nm pulse followed by 620 nm, and then 830 nm. This could persuade a reaction at the end stage (in the "bimetal core" with 660 nm, see below), to create an "open slot" (electrostatic pull) for an electron from the "previous" metal atom (heme A) so that the next wavelength can be better utilized to activate (620 nm). The 850 nm pulse would be creating an open slot (electrostatic pull) for the rate-limiting cytochrome c to insert the next electron into CCO. (( BTW, this can cause an electrostatic pull that transmits back up the entire electron transport chain to the other complexes which independently transfer another 4 H+ per CCO cycle. This can prevent electrons from "leaking" and thereby causing free radicals and possibly enable more efficient "fat burning"....if someone exercising at the same time. )) Getting the timing for each pulse and off time is very complicated since there are still too many unknowns in CCO activity. The best off time between the 670 nm and the 620 nm will be difficult to determine, but 620 might be simulatneous with 850. It might turn out to be best to apply all three at the same time because the 2009 article below says the heme a to a3 rate-limiting step may coincide with a cyt c to CuB rate lmiting step. The rest of this article will discuss more details in case an expert can figure out the exact electron movement timing and knows how long to do each pulse of each wavelength and in what sequence. Can I actually count the number of photons entering the body to determine if it theoretically corresponds to an increase in ATP that is enough to heal? Yes. At 60 kJ/mole in situ (not 30 kJ/mole) ATP->ADP releases 5E-20 joules per ATP (about 140 moles ATP per day). For a 2,000 kcal diet and 1.75 m^2 body surface area, this turns out to be 5E16 ATP conversions (5E16 electrons) per second per cm^2. Light can reach only about 10% of tissue, so where light can reach there are 5E16 * 0.1 = 5E15 electrons per second per cm^2 needed to produce normal amounts of ATP. 30 mW/cm^2 light has 100E15 photons per second per cm^2, so it has a potential of providing 20 times normal cell ATP production while the light is applied. But about 95% (19 out of 20 photons) is wasted in skin and other absorption. So 30 mW/cm^2 can only bring injured cells up to normal respiration while the light is applied, which I know from experience requires about an hour to see healing effects, and this personal observation is in agreement with the 4 to 6 J/cm^2 research: 30 mW/cm^2 * 0.05 transmittance * 3600 seconds = 5.4 J/cm^2. It is no surprise that bringing injured cells up to normal respiration for an hour would have substantial benefit. But I know from experience on many pains over the past 10 years that 180 mW/cm^2 gets the same benefit in 5 to 10 minutes, indicating that it is possible to speed up injured cell respiration rate by at least a factor of 6. Please review the "How does it work?" section if you don't understand what the following images are referring to. This is a very complicated subject that is meant for molecular biologists well-versed in light-activated electron movements, but I would like to explore the subject because of the enormous potential I've mentioned above. The CCO is a ~10-stage mechanical pump that receives energy to change its shape in response to the individual photons coming in. Individual photons from these wavelengths are converted into noticeable mechanical energy. CCO is operating sort of like a fuel cell in cooperation with the rest of the electron transport chain. Its function is to accept electrons to pump H+ to a higher concentration area while converting O2 to to 2H2O. The H+ concentration is not burnt directly, but converts ATP for burning. The presence of an electron pulls an H+ into the pump without reacting with it. An O2 to 2H2O conversion occurs in the pump, using the electrons to reset the pump to the initial state after four electrons have come into it and four H+ have been pumped into the higher concentration area for 4 ATP production. The H+ naturally exists in water plentifully at pH=7 and is continually replenished by the Krebs cycle that produces NADH that the other parts of the electron transport chain use to release the H+ from the NADH and feed in the electrons while generating NAD+ and H+. (and FADH to FAD+ and H+). The 4 H+ stay inside the pump until a cycle is completed and 2H2O is created. I beleive the 4 e- are used for created 2O-2 from O2 so that the O2 to 2H2O electron requirements balance out. 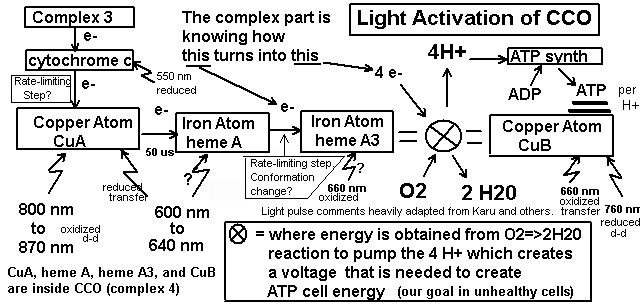

It appears an electron and H+ come into the heme A3 and CuB bimetal core at the same time, roughly being attracted to each other. The pumping action of finally getting "that" H+ to the outer membrane is triggered by another H+ coming into CCO which cancels a negative charge in the bimetal core which releases an electrostatic pull on the "to be pumped" H+ that was being held in place. 2013 from a new CCO paper: Reduced CuB receives O2 and then 4 e-, ultimately from cyt c, whose introduction to the bimetal core corresponds to pumping an H+ to the outer membrane. The 4 e- correspond to 4 H+ equivalents entering the hydrogen bond network in the reduced (gained electron) state "H". CuB remains reduced during this process which prevents water from the inner mitochondria from entering which would supply more H+ for the hydrogen bond network to reach equilibrium with the inner mitochondria's H+ concentration (acidity). Looking at my 1st diagram above, this implies there may need to be four pulses of a sequence of 850 nm and 620 nm (one following the other at an unknown time pulse widths and a delay between them) for each sequence pulse of 670 nm and 760 nm. "Timely closure of the channel is critical to ensure highly efficient energy transduction." The water channel is closed when O2 moves from CuB to Fe3 as a result of Ser382 sensing protonation of the largest water cavity which is equilibrated with the hydrogen bond network. A possible O2 storage structure exists near CuB so the cavity may have an "open" and "intermediate" state to allow protons through. Fe3 has no affinity for the O2 before complete protonation of the hydrogen bond network (H-pathway). Fully reduced CCO after the last H+ pump step, the water channelis "open" and the H-network is fully deprotonated. This allows CuB to trap O2 from a storage area near the O2 reduction site. This causes the open state. The "intermediate" allows an H+ to enter the cavity which causes it to return to "open". This transition corresponds to CO-release from CuB. In the intermediate state, H+ readily enters the H-network which regenerates the open state and return of O2 to CuB from the storage location. When it is sensed the H-network has all four protons, Fe3 affinity for O2 increases as CuB affinity decreases. e- transfer from cyt c to CuA and heme a is coupled with proton pumping. e- comes from cyt c when O2 is reduced by an electron from CuB+1 after channel closure. CuB can receive only 1 e- at a time. There are four ways pulsing may help:
Designers infoSee also 850 verses 660 and pulsing for what may be a fantastic design, not to mention safety. Customers will strap LED light therapy devices to their skin with ace bandages and under blankets and pillows and will leave them there all night and the heat can't be felt. Even 30 mW/cm^2 light output arrays can cause a 2nd degree burn if the heat can't escape. So an off timer like heating pads is required. LED viewing angle does not matter if the device is applied close to the skin. Don't forget blue LEDs will injure the retina, so use the widest angle lens as possible if treating for bacteria.The following paragraph falls under a creative commons license as it was published on instructables.com on October 5, 2013, i.e., do not commercialize without permission, cite your source, and share derivations. Concerning applications to the brain: you want 830 nm (if you can get it in an array as cheap as 850 nm) and we have no idea what the best pulsing will be except my guess is 50 uS on and 250 uS off, but square wave pulses will have harmonics in the cell-phone MHz range so that daily dosages for a few months might be a really bad idea (equivalent to 1,000 cell phone hours a day?). More than 25 mW/cm^2 (80 mW/cm^2 circuit) and the circuit will need a fan. At 30% efficiency of energy input to light output gives 0.025/0.3/2 = 42 mW per LED. 1.55 V / 0.042 = * 37 mA in each LED series for 830 and 850 nm. This gives (12-1.55*7)/0.037 = 38 ohm resistor for each series of 7 LED at 12 V. At 25 mW/cm^2 in a 40% oversized helmet with LEDs pointing inward to nearly double the light intensity (cortex has 14x17 cm diameters, so LEDs need to be 20x24 cm diameters to get a doubling effect based on spherical surfaces having r^2 areas) could be 50 mW/cm^2 which at 2% transmission (assuming bald and white skin) to cortex and needing 1 J/cm^2, means 1/0.05/0.02 = 1000 second treatment (17 minutes) plus or minus a factor of two, if the pulsing does not help a lot so that it can be reduced to 5 minutes. 1/2 of a 22 cm sphere is 760 cm^2 which is 1520 LEDs (5 mm type is needed). 40 mW per LED at 1500 LEDs is 60 Watts which will need a very open design. A 60 W bulb is hot, but spread out like this, the circuit should not get hot. Bald is needed. 20 degree emission angle (+/-10) is needed. Twice as long for dark skin or hair and 4 times as long for dark hair and dark skin, as a wild guess, but with more chance for overheating. Timer to automatically shut off after 5 or 10 minutes to check scalp temperature. Heat stress warning. Infrared arrays might be dangerous to the eyes, at least as far as our legal system is concerned. You don't need to be the best or prettiest helmet. You need to be the first powerful helmet that gets internet buzz among people who have brain-impaired relatives. No one will spend over $1000 on a helmet without seeing it help people they know. Your costs for production 1500 of the 850 nm 5 mm LEDs will be will be $400. Approval in different legal systems may be difficult if you go over 25 mW/cm^2 (and thereby need a fan). Targeting tanning salons, chiropractors, and physical therapists would be a great idea: there are about 15,000 of each in the U.S. and they have conferences where ideas can spread. You'll need someone who is an enthusiastic member of and active in those communities. I am talking from 17 years of experience in watching small health-product manufacturers start up and fail, and learning from the few that succeed to the multimillion dollar level in 5 to 10 years (Mine grew along with 4 other individually owned companies). It takes 5 years of focused work for someone who is in the upper 10% of intelligence to create one of these companies with 50% chance of success. The most important step is the initial idea. The helmet is the best idea I've seen in a long time, assuming it works in dementia or is excellent in terms of increasing thinking hours per day. Designers trying to select LEDs or arrays will have trouble comparing LED brightness from different manufacturers. The plastic encasings can focus the light and make mcd ratings much higher, but the amount of light coming out is the same. A 100 mcd LED at +/- 10 degrees (20 degrees angle of output) has the same total amount of light output as a 2,000 mcd LED at +/- 5 degrees (10 degrees). The equation is: Milliwatt output of an LED = mcd / (683 x P) x 2 x pi x (1-cos(1/2 Angle of output)). Companies are not exactly consistent in how they measure mcd (millicandela) and the angle output. Be careful in determining if they are stating 1/2 angle or full angle. P is the "photopic response factor" ( graph ) that depends on the wavelength. mcd and P are only meaningful for visible wavelengths (not infrared). P=1 for 555 nm and P=0.061 for 660 nm. For infrared, the measurement has to be mW/SR where SR=steradians. SR units are the percentage of a sphere's surface area, but divide SR by 4π (12.566) to get the percentage. SR is to a sphere as radians are to a circle. To convert from angle of output to steradians, use SR = 2 x pi x (1-cos(1/2 Angle of output)). Replace mcd/(683 x P) with mW/SR for infrared LEDs. In practice all this is not very useful. You just have to buy the LEDs and compare them. All 850 nm LED lamps I've tested had the exact same efficiency. As a rough estimate, the light output energy of an LED is 30% of the input energy. Strong LEDs use 50 to 100 mA continuously. But 20 mA red LEDs can put out enough light and are very common. A good and strong 850 nm LED will use 50 mA continuously, but the device will get too hot if you pack the LEDs closely (22 LEDs per square inch for 5 mm packages) and run them anywhere near their max. 0.8 watts per square inch is the maximum energy you can apply to any device that touches skin unless a fan or heat sink is used in order to the skin temperature below 105 F (FDA guideline). Kind of like a high fever on the skin, except the blood is able to take away the heat. So at a typical spacing of 12 LEDs per in^2 (2 LED per cm^2) you can apply 66 mW per LED. That's 45 mA at 1.55 V for the common 850 nm lamp and 35 mA at 1.9 V for a good 660 nm. LED spectrums can be generated with this spreadsheet. Despite all the above, in directly measuring LED strength as described below, I measure only half the intensity reported by the datasheets. Datasheets report very roughly 1/3 of the energy input coming out as light output. I measure only half as much, about 18%. An expert on this tells me you can expect 15% to maybe 30% light ouput from LEDs used in light therapy. You can measure the light intensity of anything in mW/cm^2 or the efficiency of a powerful single LED or weak array with less than 10% error or maybe even 5% (believe it or not) by using a styrofoam cup, cocoa powder, coffee or another water-blackening agent, and a home digital thermometer (accurate to 0.1 or 0.2 degrees C), based on the heat capacity of water. The equation is: W/cm^2 = 2cm x C x 4.18 / seconds * 1.04 where 2cm is the depth of water with dark cocoa powder to make it black water, C is increase in the water's temp, 4.184 is converting from calories to Joules, and seconds is the time the light was applied (200 seconds works best for high power device, up to 600 seconds for typical low power). The 1.04 is a correction due to 4% of the light reflecting off the surface of the liquid. This assumes the array is larger than the surface area of the liquid. For LED devices too small to cover the surface of the water, multiply this W/cm^2 result by the water surface area divided by the surface area of the LED array. The styrofoam cup needs to be cut off at 3 cm and LEDs can't be too close because air currents (if not infrared radiation from the hot array) cause direct heating from the non-light portion of the energy applied to the LEDs. In practice I find at least 30 ml is needed to counter rapid evaporative cooling of the liquid. Do not take temp measurements in the Sun or while the LED device is being applied because the thermometer absorbs the light and heats up directly. Water temp must be close to room temperature, or if you want are attempting 3% accuracy chill it below air/styrofoam temp at half the expected liquid temp change expected so that some heat is gained from ambient during the first half of the experiment and lost during the second half as the liquid temperature rises above ambient. An infrared thermometer is probably best (there is heat capacity of the metal in a thermometer that would reduce your results), but the water must be stirred (quickly with thinnest possible wooden stick due to absorbing heat) because only the surface will be heated if the liquid is dark. If the liquid is not dark and you paint the styrofoam black with a marker (spray paint melts the styrofoam), you're risking maybe 20% heating of the styrofoam instead of the water. Having a 100% dark liquid causes only the surface to be heated which means a lot of evaporative error making your results low. Saran wrap or glass floating on the water stops evaporative cooling and the reflection loss is still only 4%. Saranwrap or glass above the water will cause about 12% reduction in the measured results (multiply by 1.12 to correct) but there will still be some evaporation into the air gap. For a simple red or infrared LED array running at about 30 mA per LED I get about 30 mW/cm^2 from a 4 F rise after 10 minutes. For a blue array under the same condition I get a 6 F rise. Doing everything right and the extra decimal place in the temperature appears to give accuracy to within 5%. My results are typically significantly less than what LED manufacturer's data sheets say if I try to make calculations based on their data. I know the data sheets are wrong because their data is not usually self-consistent and my measurements of Sun intensity are very accurate to the known intensity (100 mW/cm^2 when sun is > 80 degrees in the sky). The blue sky should be blocked from providing light to the measurement cup (via a tube of some sort) because reflections from the sky can provide 5 to 15% more light. For about 2 years I wondered why I could not get less than 100 mW/cm^2 in the mid-day Sun when I should have been seeing unknown losses but not unknown gains. Then someone informed me that the blue sky adds a lot to the commonly-cited sun intensity. To calculate sun intensity at any time at any location on a sunny day, use this spreadsheet. The surface of water reflects 4% of light in LED ranges.  Better methods I'll probably explore: 1) a piece metal painted black and the infrared thermometer reading the opposite side and 2) inserting a black garbage bag plastic ni the middle of clear water to alleviate two of the problems above Skin: Wrinkles, Acne, Scars, and SpotsI have historically been against the idea that LEDs might help skin for anyhting other than recent wounds. There appears to be only one good article that disagrees with me in the case of sun-damaged skin (see this). But for now, I will retain my old, skeptical comments about LEDs and skin, as can see in the rest of this section.Simple LED devices for use at home do not work on wrinkles, aging, or scars. Wrinkles are old, fixated collagen, like scars. LEDs repair recent injuries in cells that need more energy. This is the only way they work. There is no reason to believe this will reduce existing scars or wrinkles. Pictures of wrinkles before and after are not comparable because the angle of the lighting and the amount of smiling drastically changes things. I found only one journal article (see below) that indicated simple red and infrared light energy can help. Low Power Devices for wrinkles and aging:
Spots: Non-ablative devices are not as serious in terms of risk as ablative (destructive) and they may soon be as good as the older ablative techniques. The non-ablative devices usually use high-energy focused spots of laser light that cannot be duplicated by LED devices sold on the Internet. Wavelengths from 500 to 3000 nm (blue to mid-infrared) have been used, but 1000 to 1500 is being researched the most. These techniques are improving, but are still not as good as ablative. Usually, between >1000 nm and < 1500 nm wavelengths, long or short pulsed, are used to heat the water in the skin to cause heat damage to the cells. Therefore this technology is much different than the 600-900 nm healing wavelengths that the rest of this page is concerned with. Studies have used three to eight treatments typically one month apart. Cryogenic cooling may also be used to minimize harm. At Reliant Technologies, the ablative areas are a about 0.5 mm deep into the skin and twice as thick in diameter as a human hair. "Fractional rejuvenation" or "fractional photothermolysis" is the non-ablative version of the grid pattern used in ablative techniques. Fractional photothermolysis (FP) has been recently introduced as a new concept in dermatologic laser medicine. FP employs an array of small laser beams to create many microscopic areas of thermal necrosis within the skin called microscopic treatment zones (MTZ). Even though FP completely destroys the epidermis and dermis within these MTZ, the 3-dimensional pattern of damage heals quickly and with few side effects. FP is currently used to treat fine wrinkles, photodamaged skin, acne scars, and melasma. Due to its clinical efficacy and limited side effects FP has established itself in the past two years as an alternative treatment modality to the conventional ablative and non ablative laser therapy. 2007 German articleAnd here's another review from 2006: Ablative lasers (CO2 and Er:YAG) provide the greatest improvement in photoaging, but significant adverse effects limit their use. Nonablative lasers have reduced adverse effects, but limited efficacy. Fractional photothermolysis (FP) produces arrays of microscopic thermal wounds called microscopic treatment zones (MTZs) at specific depths in the skin without injuring surrounding tissue. Wounding is not apparent because the stratum corneum remains intact during treatment and acts as a natural bandage. Downtime is minimal and erythema is mild, permitting patients to apply cosmetics immediately after treatment. As with other nonablative laser modalities, multiple treatments are required. FP represents an alternative for treatment of dermatologic conditions without the adverse effects of ablative laser devices and can be used on all parts of the body. FP can be used for the treatment of facial rhytides, acne scars, surgical scars, melasma, and photodamaged skin.To quote an outdated 2002 MedScape article to show the initial skepticism of non-ablative techniques 6 years ago: Unfortunately, clinical data in support of nonablative lasers and light sources [including LED devices] for wrinkle and acne scar treatment remain unimpressive. Despite a series of lectures and dozens of research presentations dedicated to the subject, results at this year's ASLMS often failed to impress the audience. Some before-and-after slides elicited puzzled expressions, while others triggered sporadic laughter. As one attendee murmured during a presentation, 'I can't tell any of the befores from the afters.'Since this quote, many postive articles have been published. One study used 14 J/cm^2 with a 0.3 ms short pulse at 1064 nm for improving scars. Another used a combination of blue and infrared: 7 to 15 J/cm^2 with 7 to 50 ms pulses at 535 nm and 24 to 30 J/cm^2 with 30 to 65 ms pulses at 1064 nm. 1300 nm and 1500 nm lasers are also commonly used.
Treating the RetinaRead this section at your own risk. I am not a doctor. The safety issues are complex as can be seen in the safety section below and I often make mistakes.LED light helps only cells that are injured, not dead cells nor scar tissue. Red and infrared LED arrays may help macular degeneration, retinal tear, and laser burns, but very probably not floaters. They could possibly make floaters worse. They are best at treating injuries that have occured within the past few days (the sooner the application, the better). This means about 300 seconds of a typical 25 mW/cm^2 array which is the max officially allowed for infrared before it supposedly heats the lens and cornea. I could not find any information that indicated any normal 5 mm type LED in red could ever hurt the lens or cornea, so you can apply red for longer time periods, but because the pupil response is to become smaller, red is blocked about 5 to 10 times more than infrared, which means it would need to be applied 5 to 10 times longer. My experiments indicated (see pictures below) 5 times more because the area of a 5 mm pupil is 5 times more than a 2.3 mm pupil. I have applied 150 mW/cm^2 850 and 830 nm for 3 minutes several times and did not notice any harm, which is 3 times more than is needed to treat the retina. I believe the ideal array for treating the eye will be 25 mW/cm^2 at 830 nm for 300 seconds while being moved around (definitely not kept still where the retina might be heated too much). 850 nm may work just as well. See the Safety section below on "Retinal Heating" for more information on trying to treat the retina. Especially example 4.    Safety ConcernsHalogen lights contain a lot of blue light and are very dangerous to the eyes.Typically, there are no safety concerns for LEDs unless they are blue or they are really strong or applied directly to the eye. Heating the Skin Leaving LED devices on too long and wrap tightly against the skin is probably the most likely cause of harm. Skin temperature should never be more than 41 C (105.8F) to meet FDA regs. Even a weak LED array will cause 2nd degree burns if it is wrapped snuggly with an ace bandage under blankets because the heat is not being allowed to escape. No matter how "cool" a heat-producing device operates, if it's wrapped good enough and long enough, it can get hot. I've found around 0.7 Watts per square inch to be the maximum energy that can be put into a device that touches the skin without a fan or special heat sinking, and even then it will cause 2nd degree burns if there is not an automatic off at 15 minutes and it is wrapped tightly and left in place for over an hour. Eye Safety Strong blue and white LED's harm the eyes! Strong green LEDs have 1/15th the risk of blue. Strong and focused Red and yellow LEDs appear safe for at least 5 minutes, as long as they are not the really strong kind like 1 watt and applied directly to the eye. The ACGIH does not have a safety factor specifically for simple LEDs (as of the last booklet I have which is 1996) because they appear very safe. But it has guidelines to prevent damage to the lens and retina from any light source. The TLV for laser light doesn't apply to non-laser LEDs because lasers are different because they focus the light energy in one spot which is much more likely to cause harm. Blue LEDs may harm the eyes from a photochemical injury called the "blue light hazard" (aka "solar retinitis") that can cause temporary or permanent loss of vision wherever the blue light strikes the retina. This is very dangerous and blue LEDs as key rings should be against the law. Blue is 1,000 times more dangerous than near-infrared of 890 nm (see pdf below, page 5). Red, yellow, and green also have photochemical risks, but only green has the remote possibility of causing harm (if it's high power with a narrow emission angle). Beach and snow always need dark sunglasses for protection against UV and blue damage, and over the long term it helps prevent cataracts from the infrared wavelengths. UV is the most dangerous because it has stronger photons than blue and violet. Red, yellow, and green are very safe as long as the power is not so high that the retina heats up (this may cause floaters or other less serious "silent" injuries, but I do not have a erference). For yellow, green, and most reds, it will be clear the light is too bright before any harm occurs, unless it is laser light. Do not assume any of these statements are true when dealing with LEDs. Use this information your own risk. Lens and Cornea Potentially Harmed by InfraredCataracts are the more likely source of harm from non-laser, non-blue light, and it occurs from near-infrared and far-infrared (> 770, but < 3,000 nm), but not red. If you work outside or in front of an oven all day for years without eye protection, you are slightly more likely to develop cataracts (in the lens). See long quote below. Glass and thick plastic (safety glasses might be too thin) block far infrared (steel workers) and sunglass for the beach and snow that are not too weak and orange-tinted will probably be OK for the red and near-infrared heat energy. The ACGIH TLV guidelines say infrared eye exposure to prevent injury to lens and cornea should be less than 10 mW/cm^2 if it's greater than 15 minutes. For less than 15 minutes, the TLV guidelines says mW/cm^2 should be < 1800 mW* (seconds)^(-0.75). This equation means that for a typical 25 mW/cm^2 infrared LED device, it's safe to apply up to 300 seconds. These equations overstate the risks of 770 nm to 850 nm from LEDs because the lens and cornea in this range absorb roughly 5 times less energy than longer wavelengths (see links above). Similarly, water and plastic do not hardly block 770 to 850 nm compared to > 850 nm (see charts above). This means 770 to 850 nm make it to the retina seemingly as well as red, which means, like red, they have less energy that can harm the cornea and lens. Red carries even less risk for the cornea and lens. The cornea is the outer covering of the eye that has a bump. It is most sensitve to far infrared such as facing a fire (> 1,300 nm). Closed eyelids protect it. For comparison, staring down at perfectly white beach sand filling your field of vision at noon emits about 25 mW/cm^2 in the near and far-infrared. This is very close to what a typical infrared LED array will emit (including infrared security camera night illumination arrays and infrared flashlights). Devices with fans can acheive 200 mW/cm^2 (like devices I've made for myself: see picture with blue-plastic LEDs way above). I meantion the beach and snow infrared intensity (not counting their UV's and blues) to show that the guidelines indicate they are dangerous even in the infrared, but also to remind you that blue and UV is more dangerous, and to show that the guidelines give more protection than being on the beach for 10 minutes.Near-infrared thermal hazards to the lens (associated with wavelengths of approximately 800 nm to 3,000 nm) with potential for industrial heat cataract: The average corneal exposure to infrared radiation in sunlight is of the order of 1 mW/cm^2. By comparison, glass and steel workers exposed to infrared irradiances of the order of 80 to 400 mW/cm^2 daily for 10 to 15 years have reportedly developed lenticular opacities (Sliney and Wolbarsht 1980). These spectral bands include IRA (700-1400 nm) and IRB (1.4 µm-3.0 µm). In contrast to blue light, IR-A is very ineffective in producing retinal injuries (Ham, et al., 1982, 1976). The American Conference of Governmental Industrial Hygienists (ACGIH) guideline for IRA exposure of the anterior (front) of the eye is a time-weighted total irradiance of 10 mW/cm^2 for exposure durations exceeding 1,000 s (16.7 min) (ACGIH 1992 and 1995). Pitts, et al. (1979) showed that the threshold radiant exposures to cause lenticular changes from IR-A were of the order of 5,000 W*s/cm2. From Vishay "eyesafe.pdf" document IEC 62471 AND EU DIRECTIVE 2006/25/EC: For all applications the standard IEC 62471 is applicable. This standard for incoherent sources replaces for LEDs the laser standard IEC DIN EN 60825-1. In case of IR - Emitters the dominating limit is the cornea/lens risk in the wavelength range from 780 nm to 3000 nm. This limits the irradiance to Ee = 100 W/m2 [10 mW/cm^2] which is expressed as [radiant] intensity a value of Ie = 4 W/sr with the measurement condition of that standard with 0.2 m distance in mind (Ie = Ee x r^2). Evaluating the other limiting conditions as the thermal retinal risk and blue light hazard result in not limiting higher values for wavelengths > 850 nm and therefore are not to be taken into account. Only for 830 nm a little reduction to Ie = 3.77 W/sr is given by the thermal risk. This is still far above of the emitted intensities of IREDs covered by the Vishay datasheets.10 mW/cm^2 is the main number of interest which means 850 nm arrays might be dangerous. A single 5 mm 850 nm LED can emit 15 mW without any special cooling and up to 100 mW if cooled and driven at 2x it's 100 mA specs (assuming emission 30% efficiency), decreasing its lifespan. The cornea and lens are less than 1 cm^2, so the 10 mW/cm^2 limit can be exceeded with just 1 5 mm LED that is within 2 cm of the eye. It seems like Vishay made a mistake by only looking at the standard in terms of 4 W/sr which assumes a distance of 20 cm. The standard at 1 cm would be 100*(0.01)^2 = 0.01 W/sr and all of their 5 mm infrared LEDs will do that instead of "none" like they seem to claim above. Their wording above is tricky. You might conclude they were saying "our products are safe" but they did not, and they switched from talking about irradiance to intensity. Retinal HeatingSorry, but this is a long and difficult section and it may not have any importance at all unless you're researching retinal exposure for healing and safety. Everyone else: it's almost assuredly safe, so please skip this.This is not an issue for most red and near infrared LEDs, but I need to go into detail because people are applying LEDs directly to the eyes and the information is hard to find, even harder to understand, and this is the only page that shows how to apply the equations to an infrared array (it took me several days to figure it out and type everything below). The retina can be harmed by all visible light and near infrared, but not by far infrared that is blocked by the lens, cornea, and liquid in the eye. Internet references and even books are very poor at explaining the light intensity on the retina, so I'll go into painful detail. There is a discrepency on basics like the eye's focal length. Is the focal length 1.7 cm or 2.2 cm? It's not fixed like most web sites assume because it increases from about 1.7 to 2.2 cm as you focus on objects further away, not counting differences for different size eyes (although different size eyes also have larger pupils so the effect cancels). If you're over 40, about 20 cm is about your shortest focus which gives focal length = 2.2 as lowest value. It's important, because (2.2/1.7)^2 in the equations means 67% higher intensity on the retina if it's 1.7. Someone really near-sighted might have a focal length of 1.4, if I understand it correctly, which would be 2.5 times more light intensity on their retina than others for some of these calculations. Pupil radius increases the light intensity on the retina by a factor of 12 as it changes from dark indoors (0.7 cm) to bright outdoors (0.2 cm), but when I applied 850 nm infrared devices which show a slight red glow (see pictuer above), my retina dilated to only 0.53 cm, which is the max I'll use in the equations. Equations All the following assume the light rays are not parallel or converging, but diverging. Steridans measure the "AREA" of a sphere's surface in the same way degrees measure the "LENGTH" of a section of circle. There are 360 degrees in a circle and 4*pi steradians in a sphere. Mathematically: Steradians = 2*pi*(1-cos(1/2 angle of emission)) = 4*pi*[sin(1/4 angle of emission)]^2 and for small angles = pi*(half angle in radians)^2. Angle of emission in radians as seen at a distance = diameter of source/distance to source. X = radiance in mW/cm^2/Steradian where the surface areas we'll use for the light source and the receiving object are facing each other and not tilted (so a cosine angle adjustment is not needed). (1) X = (mW of light source towards receiver) / (cm^2 area of the LED die or light filament as *observed* by the receiver) / (emission angle in steradians towards receiver of the given mW) (1b) X = (mW of light source in beam) / (steridians of beam angle) A couple of my examples below did not benefit from knowing 1b when I wrote the examples. X is "radiation intensity" or "spectral radiance" and is very confusing, but very useful because it is the light "radiant flux" (watts) through a surface area that does not change as you get further from the light source, so it is a wonderful constant for a given light source. It is irradiance ( W/m^2 or mW/cm^2) divided by the steridians of the meters^2 at where the watts were measured. The steridians of the meters^2 measurement location depend on how far the measurement location is from the initial source of light. You can't measure mW/cm^2 some arbitrarily chosen distance from the LED and then divide by the constant steridians calculated from the emission angle reported on the LED data sheet. You use 1b above, despite my complicated example below. The reasoning to explain why it works is difficult (for me, at least). (2) X = (mW emittedl) / (cm^2 *apparent* size of the light source) / [ 4*pi*(sin(1/4 of emission angle of the mW))^2 ] angle of emission = 2*arctan(diameter of a distant cm^2 the light passes through / 2 / distance to light source) (3) X = (mW / cm^2 ) * 1/pi * (distance from source)^2 / (radius of source)^2 assuming the source is small such that sin(radians)~radians and mW/cm^2 is measured at the "distance from source". (4) (diameter of spot on retina) = (diameter of object) * (inside eye diameter = 2.5 cm) / (distance to object) (5) 1 / (focal length) = 1 / (inside eye diameter = 2.5) + 1 / (distance from light source to pupil) Assume (inside eye diameter) = 2.5 cm eye focal length ~ inside eye diameter ~ 2.2 cm unless it is a young person focusing close, or someone really near-sighted. Not 1.7 cm which is "object focal length". "Angular subtense" of object being viewed = degrees in field of view = 2*arctan(height/2/distance) k = fractional amount of light the eye does not block before getting to retina. About 0.90. (6) mW/cm^2 on retina = X * pi * (pupil radius)^2 / (focal length)^2 * k (7) mW/cm^2 on retina = mW/cm^2 * (area of pupil) / (area on retina the light is striking) * k (8) mW/cm^2 on retina = mW/cm^2 / [ 4*(sin(1/4 emission angle))^2 ] * (pupil radius)^2 / (focal length)^2 * k (note: pi in numerator and denominator cancelled) (9) mW/cm^2 on retina = mW light / (apparent cm^2 area of the LED die or light filament) / (emission angle in steradians) * (pupil radius)^2 / (focal length)^2 * k (10) mW/cm^2 on retina = mW of laser / area of spot on retina = mW / (pi * (1/2 radian divergence of beam * eye focal length)^2). This simple last equation works because laser enter in an area that is smaller than the pupil. Note: given a die size and the watts emitted from the LED, I could not find a way to properly estimate the important X in mW/cm^2/sr it is emitting. For example, say a 0.01 cm^2 die is emitting 300 mW light (i think this is about right for some 1 W LEDs possibly being run a bit hot - the manufacturer maybe should have used a larger die). Right at the surface I can estimate 1/3 of the 300 mW is coming out the front of the die directly, and the steridians for that "half sphere" is about 6, so this gives 100/0.01/6 = 1,700 mW/cm^2/sr, but I don't know how accurate this is, and I don't know how to do it more accurately. It might be off a factor of 2. If the die were a sphere with 0.02 cm^2 surface and emitting 300 mW, then it would be easier: 300/0.02/(4 pi steridians) = 1,200 mW/cm^2/sr. A flat die seems like it should be a little better at directing in a more forward angle, so my 1,700 makes sense. LED lenses seem to not increase mW/cm^2/sr as much as you would think (and maybe not increase it at all) because they increase the apparent size of the die which increases the cm^2 in the denominator even as the mW being directed into a tighter angle is increased. Example 0: blue light hazard You have a 460 nm 5 mm blue LED that has a 15 degree emission angle and are operating it at 30 mA and 3.5 volts. It is believe to be about 40% efficient in terms of Watts of light output per Watts of eletrical input. How long can you safely stare at it? Answer: (I have not checked this for accuracy, it could be wrong) The 2002 NCGIH TLV safety rule for the blue light hazard is seconds max should be less than 100 J/(cm^2*sr) per W/(cm^2*sr) per (an adjustment factor) of the light source. The total light output is 0.03 A * 3.5 V *0.40 = 42 mW. The TLV boobklet says 460 nm is nearly twice as dangerous as 480 nm (and has less than 1/2 the eye's response per mW/cm^2 as 480 nm, so a person will not sense the danger as he could with the Sun) so the adjustment factor is 0.8 for 460 nm instead of 0.45 for 480 nm. The steridians for a 15 degree full angle is 0.0537. So 100 / (0.042 / 0.0537 *0.8) = 102 seconds. More than 1.5 minutes of this LED may cause permanent damage to the retina. The closer to this LED, the larger the damaged area of the retina. Example 1: Take a typical 5 mm type 850 nm infrared LED that outputs a typical 15 mW of light from 50 mW power input. Its plastic case is a little sharper than many, outputting 75% of the light energy into only +/- 10 degrees. What is the mW/cm^2 of the light on the retina at 1 cm away from the eye? At 10 cm? How big is the image created on the retina? How many cones is it hitting? To answer this, I first need to know how big the LED light source APPEARS after being focused. The die in this LED measures about 0.033 cm on a side which is 0.001 cm^2 area, but this is not what I need (BTW, 100 W/cm^2 die seems to be what a lot of LEDs can be operated at, with 1,000 W/cm^2 peak pulses). The area it APPEARS is a function of how much it has been focused, and I don't know to calculate that from the emission angle. And the LED die sits in a bowl reflector that is reflecting the sides and bottom light from the die. So instead of trying to use guesstimate math, I'll use guesstimate observation and take a picture of this LED in operation (see below, note: total area is 0.055 cm^2, not 0.10, even though I crossed it out). The light shows a lot better in the camera than the dim red that you can see with the naked eye. The radiant intensity is: Eq (2): X = 15*0.75 mW / 0.055 cm^2 / ( 4*pi*[sin(20/4)]^2 ) = 2145 mW/cm^2/sr Assume a typical focal length of 2.2 cm (it's not someone under age 35 who is focusing less than 20 cm nor someone strongly near sighted) and a dark room to let more LED light in so pupil radius = 0.6/2 cm. Eq (6): mW/cm^2 on retina = 2145 * pi * (0.3)^2 / (2.2)^2 * 0.90 = 110 mW/cm^2 at all distances, which provides a nice 6 J/cm^2 treatment in 55 seconds. Width on retina of the image at 1 cm and 10 cm: eq (4): width = 0.16 cm * 2.5 / 1 = 0.4 cm eq (4): width = 0.16 cm * 2.5 / 10 = 0.04 cm A length of 0.04 cm * 3,800 cones/cm = 150 cones Notice image size on retina is the only thing that depends on distance. So the image gets smaller as the LED is further away, but the intensity of light per cm^2 does NOT. So if the intensity were dangerous to individual rods and cones like blue or UV light, then it does not matter how far away you get, cells would still be injured. This is long as the diameter of the image on the retina is larger than 1 rod or cone (a 0.2 cm arch weld from eq (4) covers 1 cone at 66 feet but I have not verified this). 
Example 2:This is a lengthy and wide-ranging "example" is more of a discussion and illuminatino of complicating factors that ends with an example. My infrared LED emits from the front surface the 5 mW per 0.033x0.033 cm die. 5 mm LEDs can often be pulsed with 10 times higher amps with a 10% duty cycle (others like SMD and super bright can pulse only 60% higher), but the rods and cones are not likely to know the difference with pulsing because they require heat build-up to "melt" just like the semiconductor, so it balances out in terms of safety (and how strong the light appears). It appears 1 W LEDs have dies about 0.1 cm wide and at 30% efficiency, they might be emitting 300mW/0.1^2 = 30,000 mW/(die cm)^2. Coming directly out the front surface might be half of this, 15,000 mW/cm^2 die, which is 3 times higher than my infrared LED. This makes sense: the 5 mm LEDs can't be run as intense continuously per die area, but can be pulsed higher. This implies the 5 mm LEDs can't dissipate heat as fast and that the peak pulse of amps depends on the die area. So it appears the 50 mW 5 mm LED and the 1,000 mW white LED can reach similar max temperatures and similar max peak watts per cm^2 of die area. This has importance in trying to understand generalizations about the safety of LEDs in terms of thermal effects on the retina (I will not discuss blue hazard because it is more clearly dangerous and things are complicated enough trying to determine red and infrared safety). Getting back to this "example", let's assume no LED plastic cover is converging the light on a spot smaller than the die area itself but only directing the light forward that would normally get lost to the sides. So at max it is a perfect laser who's beam is not diverging even a little like normal lasers. LED focusing can't be nearly as good as lasers and even lasers can't be exactly parallel rays, but I want to look at most intense case for LED dies. Let's say the die is infinitely wide and tall and there's no focusing (rather, instead of focusing to make it equal to the worst case of non-converging focusing and easy to calculate). This is a "worst case" for safety by trying to make it maximum intensity. Focusing can't be stronger than this unless the focusing is converging the rays onto a point rather than simply directing the light in a more "forward" direction. This does not increase the intensity of the light per cm^2 PER DIE AREA as it appears on the retina because that type of focusing (non-converging) makes the die APPEAR bigger as in example 1 (as the mW increases from focusing, the apparent die aera increases, so there is no net radiant intensity increase: mW/cm^2 of die area I think might be a constant). So an LED casing is more like a microscope which needs more light on the object to see it because the objective lens is small compared to your pupil but not like a telescope which has really large optics to focus DOWN the light into your pupil. Getting back to this example's theoretical infinitely wide and tall die, there is a rule that says for a flat plane emitting any type of rays per cm^2 like light, sound, or gravity the strength will not diminish over distance. This is because as it tries to get weaker with distance, contributions from the sides increase to balance it out. So the mW/cm^2 of the die will be the mW/cm^2 that your pupil sees. Let's guess that no commerical LED die constantly emits more than 30,000 mW/cm^2 per die area, and so the pupil will see 30,000 mW/cm^2 (no steridians in denominator because I tricked it to go away with an infinte die) from this huge die. This is 300 times the strength of the Sun but it covers the entire the font field of vision rather than a single spot like the Sun. The Sun is 0.53 degrees instead of 180 degrees which is 100,000 times less surface area (steridians) of the emission source. So, the die is 300 times stronger mW/cm^2, but coming from 100,000 times more surface area, giving 300/100,000 = 0.003 times less "radiance" (radiant intensity mW/cm^2/sr) that is important in terms of intensity as a spot on the retina. Said another way, this very large LED's 30,000 mW/cm^2 hits the pupil, and it SPREADS OUT across the much larger retina area. The pupil's hole is at most pi*(0.7/2)^2 = 0.38 cm^2 (infrared LEDs would allow this) letting light through and the retina is about 13 cm^2, so the average intensity should be roughly based on this ratio of areas: 30,000 * 0.38 / 13 = 900 mW/cm^2 on the retina. More precisely, using the focal length and equation (8), I get 341 mW/cm^2 so the method of simply dividing by the area of the pupil by area of retina is a 2.5x over-estimate. Eq (8) for the Sun's 100 mW/cm^2 gives 106,000 mW/cm^2 spot on the retina. Dividing 341/106,000 = 0.003 less from the LED as predicted above. But using a better number for the pupil radius as in the next example gives a much lower 8,700 mW/cm^2. A small change in pupil radius can have a large effect because it is a squared factor. 8,700 / 341 = 25, meaning the Sun is roughly 25 times worse on a spot of retina than the most massive and dangerous red or infrared LED I can imagine. Since the small spot from the Sun dissipates heat better, the safety difference is actually much less than 25. Once the correction for "retina heat dissipation" is made, such a large LED die is more dangerous than the Sun, and for the entire retina, not just a spot. Example 3: Sun, eye focused at a distance (focal length=2.2 cm), bright outside (pupil radius = 0.2/2). Sun insolation is 100 mW/cm^2 and is 0.53 degrees in the sky: Eq (8) mW/cm^2 on retina = 100 mW/cm^2 / [ 4*(sin(0.53/4))^2 ] * (.1)^2 / (2.2)^2 * 0.9 8,700 mW/cm^2 This heat energy is the upper bound of what is safe for 1 second exposure. The real danger from the Sun is the blue and UV which are not part of the considerations in this section. We are designed to cope with the Sun in our peripheral vision for most of the day. This gives an idea of how safe red and infrared LEDs. Example 4: Apply a 25 mW/cm^2 from a typical 850 nm infrared ARRAY to the eye in a dark room. In order for this calculation to be more accurate, the array needs to be far enough away that the LEDs appear similar in strength which indicates their light beams are overlapping. If the array is close and the emission angle is small, then only 1 LED will appear bright for a very small section of retina and you would just use example 1 for that 1 LED. For an array, you are not getting a nice and even mW/cm^2, but you get an array of spots on the retina where each spot corresponds to example 1 (75 mw/cm^2 per spot). So to get an even treatment, the array should be moved in tight little circles. The tighter-angle LEDs will have to be further away to appear the same strength, which means the array has to be wider to cover a large area. The following is for 25 mW/cm^2 850 nm array, at +/- 15, +/- 10, and +/-5 degrees emission angles, a dark room (0.6 pupil), and not trying to focus at a close distance ( 2.2 cm focal point): Eq (8) mW/cm^2 on retina = 25 / [4 (sin(5*2/4))^2 ] * (0.53/2)^2 / 2.2^2 * 0.9 = 43 mW/cm^2 Eq (8) mW/cm^2 on retina = 25 / [4 (sin(10*2/4))^2 ] * (0.53/2)^2 / 2.2^2 * 0.9 = 11 mW/cm^2 Eq (8) mW/cm^2 on retina = 25 / [4 (sin(15*2/4))^2 ] * (0.53/2)^2 / 2.2^2 * 0.9 = 4.5 mW/cm^2 These numbers are only if the array is being moved around in the field of vision. If stationary, then use example 1 for each spot the array creates on the retina. The 11 mW/cm^2 for +/- 10 degrees is 10 times less than for a single LED kept still as shown in example 1. This implies the "no-light" area where there is not LED light from the circuit board as seen by the eye is 10 times the area of the light seen coming from the individual LEDs. In example 1 I observed the LED light per LED covering 0.055 cm^2. I use these LEDs to produce 25 mW/cm^2 arrays and I space them 2 LEDs/cm^2. So the light is coming from 2 LEDs (0.11 cm^2 of light) per 1.0 cm^2 of circuit board space, a factor of 10 difference, right in line with these calculations. For a red LED, the pupil will be 0.23 cm instead of 0.53 cm giving: 7.4, 1.9, and 0.8 mW/cm^2 which is very small. If I were treating my retina I would use an infrared array with +/- 5 or +/- 10 degrees LEDs. Treatment time is to get about 6 J/cm^2. For example, at 25 mW/cm^2 with +/- 10 degree LEDs, retina gets 11 mW/cm^2 which would be 6/0.011 = 545 seconds = 9 minutes as a max, maybe 3 minutes as a minimum that may be enough to get good benefit. More than 10 minutes may start reducing the benefits. According to the TLV safety guidelines below, more than 5 minutes is not safe for the lens and cornea at 25 mW/cm^2 at 850 nm, but I believe they are being too strict for this wavelength as detailed above. It will be hard to know how many mW/cm^2 an array is supplying unless an experienced person looks at the operating array, or if the manufacturer actually knows his specs rather than guessing from data sheets. 25 mW/cm^2 will be what a good security camera illuminator array from ebay will provide, given that they use 5 mm LEDs spaced about 2 per cm^2 and you can easily see the red glow from the 850 nm LEDs in a lighted room. There is a section above that shows how to measure it if the measurement is done carefully and correctly. The retina does not block much infrared light according to one source, so the back of the retina is being treated too. Clearly retinal tears and laser burns within the past day or two need to be getting infrared treatments before the cells die. Anyone else copying what I would do for myself assumes full responsibility for the results. Retinal Safety Calculations The ACGIH TLV safe dose for for the retina for green, yellow, red, and near infrared is anything that causes one of the following to be less than 1: for 1 us to 10 seconds: 1/5000 * X * (width of source) / (distance to source) * seconds^0.25 The above is for 500 nm to 700 nm thermal only. I won't discuss blue light hazard which is a different topic. for 700 nm to 1050 nm multiply by 10^[(700-nm)/500] for > 10 seconds: 1/600 * X * (width of source) / (distance to source) this second one is based on a 7 mm pupil and a detector field of view of 11 mrad (i don't know what that means). At 10 seconds this seconds equation comes out about 8 times more dangerous than the first. There is another equation for cornea and leans safety, but it seems to be 10 or 100 times "safer" (less likely to affect cornea and lens) at less than 100 seconds, and many times more dangerous at 1000 seconds or greater (see previous comments). The "width/distance" is a factor that adjusts for smaller spots on the retina which are less dangerous because the heat can spread better to the sides of the retina, as long as the spot is less than 1.7 mm. If the spot is larger, this ratio is no longer has an effect. 1.7 mm spot is supposed to be 0.1 radians (source of light has a diameter 1/10th the distance to the eye), so that for ratios larger than 0.1, make the ratio a constant of 0.1 rather than further penalizing the source. However, I did not see where the ACGIH says this and even gives an example using 0.5 radians. So maybe the ratio is not meant to show small spots are safer, but to show larger spots are more dangerous. The danger to the retina could be thermal burns that do not heal (blindness in spots) or maybe more floaters obscurring a little bit of vision from the internal fluid pulling away from retina a little more. Example 5: (most dangerous red/infrared LED example) For 10 seconds apply 1 Watt 660 nm or 630 nm LED outputing 75% of its 300 mW within +/- 20 degrees at 1 cm in front of pupil . Assume die area is 0.1x 0.1 = 0.01 cm^2 but that the focusing lens makes the die appear 5 times wider. 1/5,000 * 300 mW*0.75 / (4*pi (sin(20*2/4))^2) * 0.1*5 / 1 * 10^0.25 = 0.066 (supposedly safe by 1st calculation) 1/600 * 300 mW*0.75 / (4*pi (sin(20*2/4))^2) * 0.1 / 1 = 0.31 (supposedly safe, but not. See below) If the angle of emission is +/-10 degrees, then multiply these numbers by a factor of 4, so the bottom is unsafe. Actually, since there is focusing into a small angle, the LED die will appear larger than it's actual 0.1 cm width, so these numbers are probably not safe (the 0.1 number should be larger, maybe 5 times larger). Example 6: Typical 850 nm 5 mm LED safety. Using data from example 1, hold a 850 nm LED 1 cm from pupil for MORE than 10 seconds: 1/600 * 2145 mW/cm^2/sr * sqrt(0.055) /1 = 0.82 This gives < 1 therefore supposedly safe for any length of time. Looking at the LED this "apparent die size after focusing" is not "contiguous" (there are gaps between the light) so that the retina may be able to dissipate the heat better than I'm implying, so it should be safer than this by a factor of about 3. Manufacturers may desire "contiguous" light from the focusing lens but this shows that is not desirable from a safety standpoint. An array of these will not be more dangerous than the individual LEDs, but simply affecting more spots, if it does have an effect. However, 5 mm LEDs can be run at 2 or 3 times than their ratings for 10 seconds without burning up. If they are kept cool with a fan they will still be efficient in turning the energy into light. So it is possible to run a 5 mm red or infrared LED in a way that it is not safe to look at it within a few cm of the eye. My guess is that it would be difficult for them to be harmful at more than 10 cm from the eye. LED Array: if the array is moved around in the field of vision, then the 2145 mW/cm^2/sr is effectively 10 times smaller as described in an example above, so arrays being moved around to treat the retina should be safe. Example 7: Sun safety. 1 second staring at Sun. Sun insolation is 100 mW/cm^2 and is 0.53 degrees in the sky. X = 100 * 1/pi * (distance to sun)^2 / (sun radius)^2 = 1,460,000 mW/cm^2/sr Safety calculation: 1/5000 * X * 1.4E6/1.5E8 * 1^0.25 = 2.6 which is > 1.0 so it's risky even for a second if this is going to occur often in the work environment, not counting the UV and the much more dangerous blue light hazard. Example 8: Sun for > 10 seconds in one spot on eye: 1/600 * X * 1.4E6 / 1.5E8 = 23, very dangerours. Lasers are covered on other web pages, but here's the equation and calculation for comparison: mW/cm^2 on retina = mW of laser / area of laser spot on retina = mW / (pi * (1/2 radian divergence of beam * eye focal length)^2. Example 9: standard class IIIA, for presentations, 4 mW laser pointer, 0.001 rad divergence (I tested mine at 30 feet to confirm this): mW/cm^2 on retina = 4 mW / (3.14*(0.0005*2.2)^2) = 1,760,000 mW/cm^2 This is 200 times more than the Sun. The only saving grace is that it is usually moving fast across the eye in a very small spot so that the tissue absorbs the 4mW heat energy. The small spot getting all the energy is the problem in the first place, but my point is that if it were as wide as the Sun (8 times wider), then it would not be 200 times more dangerous, but 8 times smaller is 200/8 = 25 times more dangerous, except the Sun has blue and UV which is the usual source of harm if you don't count life gaurds on white sand beaches who don't use sunglasses. When the light beam is smaller than the pupil, the pupil radius is not in the equation. The threshold retinal irradiances for just producing visible retinal lesions in the rhesus monkey eye were approximately 30 W cm^2 for 1 s, 23 W cm^2 for 10 s, 20 W cm^2 for 100 s, and 19 W cm^2 for 1,000 s (all for 500-um retinal spot diameters). This compares to only 0.03 W cm^2 for a 1,000-s exposure to 441-nm blue laser light (WHO 1982). Thermal retinal injury has been shown to dominate at wavelengths beyond 550 nm, and the threshold for thermal injury is retinal spot-size dependent because heat flow is more efficient for smaller diameter image sizes. The 500-um thresholds for thermal injury would be expected to be nearly twice the value for a 1,000-um (1-mm) image [1mm at same W/cm^2 is twice as dangerous because heat is not absorbed as well by surrounding tissue]. The 500-um image size corresponds to an angle of 29 mrad. For still larger retinal image sizes, this spot-size dependence becomes less, and by 1.7 mm diameter, the threshold is virtually a constant with increasing spot size. The 1.7-mm diameter retinal image is approximately 100 mrad [ 1.7 cm focal length * 0.1 rads incoming light = 1.7 mm spot on retina], and this is applied in guidelines to protect against thermal injury from both lasers and incoherent sources. Since the retinal irradiance is directly proportional to 
 Examples of Journal ArticlesThis is just a sampling that I haven't updated. The literature is too vast to review."But if the rats were treated with LED light with a wavelength of 670 nm for 105 seconds at 5, 25 and 50 hours after being dosed with methanol, they recovered 95 per cent of their sight. Remarkably, the retinas of these rats looked indistinguishable from those of normal rats. 'There was some tissue regeneration, and neurons, axons and dendrites may also be reconnecting,' says Whelan."
"We believe that the use of NASA Light-Emitting Diodes (LED) for light therapy will
greatly enhance the natural wound healing process, and more quickly return the soldiers to a pre-injury/
illness level of activity. The use of LED in combat with self-healing patches in future may enable
the soldiers even after they are wounded to persist in combat better and longer."
"LED produced improvement of greater than 40% in musculoskeletal training injuries in Navy SEAL team members, and decreased wound healing time in crew members aboard a U.S. Naval submarine. LED produced a 47% reduction in pain of children suffering from oral mucositis. CONCLUSION: We believe that the use of NASA LED for light therapy alone, and in conjunction with hyperbaric oxygen, will greatly enhance the natural wound healing process, and more quickly return the patient to a preinjury/illness level of activity. "
"ATS treatments improve sensation in the feet of subjects with DPN, improve balance, and reduce pain."
"This technology may be the answer for problem wounds that are slow to heal....diabetic skin ulcers and other wounds in mice healed much faster when exposed to the special LEDs in the lab. Laboratory research has shown that the LEDs also grow human muscle and skin cells up to five times faster than normal...."
"Light close to and in the near-infrared range has documented benefits for promoting wound healing in human and animals. "
"ATS treatments improve sensation in the feet of subjects with diabetic peripheral neuropathy, improve balance, and reduce pain."
"Near-infrared irradiation potentially enhances the wound healing process, presumably by its biostimulatory effects."
" It was found that laser exposure resulted in more pronounced restoration of functional state of nervous fibers than conventional therapy. Application of laser irradiation of low intensiveness was effective while in combined therapy of distal diabetic polyneuropathy as well as monotherapy."
"exposure of volunteers to visible and infrared polarized (VIP) light leads to a fast increase in the growth promoting (GP) activity of the entire circulating blood for human KCs in vitro, which is a consequence of the transcutaneous photomodification of blood and its effect on the rest of the circulating blood volume."
"The method of monochromatic near infrared stimulation can be used for selective stimulation of several regions of the external auditory canal,.."
LED and LLL irradiation resulted in an increased fibroblast proliferation in vitro. This study therefore postulates possible stimulatory effects on wound healing in vivo at the applied dosimetric parameters.
Wound healing was significantly more rapid with than without FIR. Skin blood flow and skin temperature did not change significantly before or during far-infrared irradiation.
Although more studies are needed, LED therapy appears useful in the prevention of OM in pediatric BMT patients.
News articles on the NASA Study:
A wound-healing device was placed on the USS Salt Lake City submarine, and doctors reported 50 percent faster healing of crewmember's lacerations when exposed to the LED light. Injuries treated with the LEDs healed in seven days, while untreated injuries took 14 days. second daily infrared (JR) laser (820 nm, 25mW) and visible red laser (670 nm, 10 mW) at 1 J/cm2 and 5 J/cm2 on chronic pain. ...five treatment sessions over a two-week period. ...significant reductions in pain over the duration of the study with those groups which received infrared (820nm) laser a 1 J/cm2 and 5 J/cm2 904 nm three times weekly for 2 weeks, ......tendonitis of the shoulder 3.5-inch by 4.5-inch (89-millimeter by 114-millimeter), portable flat array of LEDs, arranged in rows on the top of a small box. ......places the box of LEDs on the outside of the patient's cheek about one minute each day. The red light penetrates to the inside of the mouth, where it seems to promote wound healing and prevent further sores in the patient's mouth. All 176 patients received six treatments during a period of 3-4 weeks. ..GAAs laser therapy for tendinitis and myofascial pain A 40 year-old woman presented at the Abe Orthopedic Clinic with a 2-year history of lower back pain and pain in the left hip and leg diagnosed as a ruptured disc between the 5th lumbar/1st sacral vertebrae. .....The gallium aluminum arsenide (GaAlAs) diode laser (830 nm, 60 mW) was used in outpatient therapy, and after 7 months, the patient's condition had dramatically improved, demonstrated by motility exercises. This improvement was confirmed by further MRI scans, which showed clearly the normal condition of the previously herniated L5/SI disc. Influence of low-level (810nm, GaAlAs semiconductor) laser on bone and cartilage during joint immobilization was examined with rats' knee model. .......The hind limbs of 42 young Wistar rats were operated on in order to immobilize the knee joint. One week after operation they were assigned to three groups: irradiance 3.9W/cm2, 5.8W/cm2, and sham treatment. After 6 times of treatment for another 2 weeks both hind legs were myofascial pain in the cervical region. The patients were submitted to 12 sessions on alternate days to a total energy dose of 5 J each. RA:From July 1988 to June 1990, 170 patients with a total of 411 affected joints were treated using a GaAlAs diode laser system (830 nm, 60 Mw C/W). Patients mean age was 61 years
890 nanometer (nm)....Venous ulcers, diabetic ulcers, and post-amputation wounds....It recently has been demonstrated that application of this particular MIRE device to the skin for 30 minutes increases plasma NO in nondiabetic subject volunteers, as measured with a Sievers Instrument, Model 280, Nitric Oxide Detector
|
Introduction Conditions Helped How Does It Work? Strength & Dose Penetration Brain Build A Helmet Advice for Common Injuries Best Wavelengths Laser vs. LED Sunlight Halogen Lights LED Beds Cancer Pulsing For Designers Skin Retina & Safety Journal Abstracts |